Aim
To better determine the impact of green peach aphid feeding damage in the absence of virus on canola yield and seed quality.
Background
Previous work has shown that yield loss caused by green peach aphid (GPA) feeding damage is minimal in canola. However, defining this yield loss has been complicated by aphid-transmitted virus, such as beet western yellows virus. It is also common for multiple aphid species to colonise canola at the same time and cabbage aphids can cause significant yield loss through feeding damage if numbers are high enough. In this trial we ensured that GPA was the only aphid species present and was devoid virus.
Trial details
Canola, cv. Pioneer 43Y23, was sown at the Department of Primary Industries and Regional Development's Geraldton research facility in Geraldton on 12 April using a plot cone seeder. Twelve insect exclusion tents measuring approximately three square metres were placed over the seeded area to ensure aphids could not colonise the canola from the surrounding area. At the six leaf growth stage, canola seedlings were thinned to ensure all tents contained 20 plants in a similar spatial configuration.
Treatments included a nil, (no aphids introduced) and three different dates of aphid introduction; 27 May (early), 21 June (mid) and 19 July (late), with four replicates of each treatment. Aphid introductions were done by placing a green leaf with 10 live aphids onto each plant within the insect proof tents. The aphids that were introduced were early were bred in the laboratory, those introduced late were collected from capsicum plants, which are not a host for canola viruses.
Aphid populations in the tents were measured by counting the number of aphids on 20 random plant leaves. This was done three times (98, 111 and 128 days after sowing) during the growing season. GPA populations were left to persist on plants until harvest without an insecticide application. Plants were hand harvested with biomass, yield components, yield and seed quality measured.
Treatments
There were four treatments based on different timings of GPA being introduced to the insect exclusion tents. This was done to create different aphid populations as described below:
- Treatment 1: Control (no aphids)
- Treatment 2: 27 May aphid introduction (early-season colonisation)
- Treatment 3: 21 June aphid introduction (mid-season colonisation)
- Treatment 4: 19 July aphid introduction (late-season colonisation)
Results
Seasonal conditions
Rainfall at the site was 392mm for the year from sowing to maturity . This compares to a long term yearly average for Geraldton of 445mm. The season was very mild with a soft finish.
Aphid populations
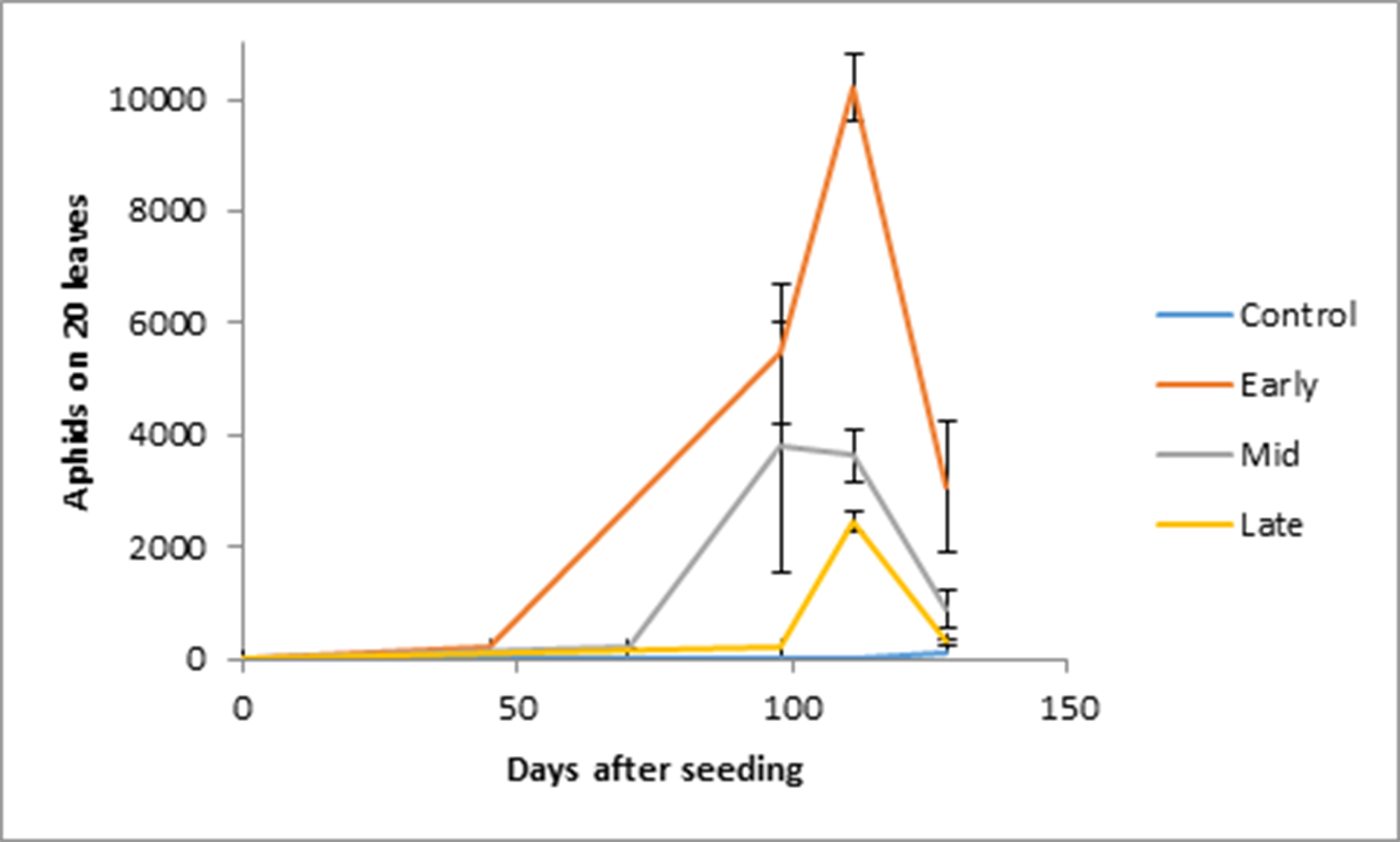
Aphid populations were highest in the tents with the earliest introduction of aphids. At 111 days after sowing aphid colonies peaked. At this time the earliest introduction treatments had aphids that were four times higher than the late introduction treatment with more than 10 000 aphids per 20 leaves (Figure 1). The controls were all free of aphids at 111 days after sowing but there was one replicate of the control with at least 100 aphids per 20 leaves at 128 days after sowing. An insecticide was applied to all control treatments tents at this time to ensure the aphid numbers were brought back to zero.
Growth and yield
The timing of aphid introduction and subsequent aphid numbers had no effect on the key measurements of growth including single plant weight, pods per plant and seed weight (Table 2). For seed weight there was a trend of decreased weight with increased aphid pressure in the early timing of introduction treatments, however this was not significant (P<0.05).
The oil content of the grain was approx. 38% and did not differ significantly between treatments, even with the very early introduction of aphids.
| Treatments | Plant weight (g) | Pods per plant | Oil (%) | 1000 seed weight (g) |
|---|---|---|---|---|
| No aphids | 97 | 513 | 38.9 | 3.8 |
| Late | 107 | 563 | 37.1 | 3.7 |
| Mid | 93 | 471 | 37.7 | 3.7 |
| Early | 91 | 489 | 38.5 | 3.1 |
| NS | NS | NS | NS |
Yield was not significantly affected by the timing of aphid introduction (Figure 2), however yield was lowest in canola plants given early GPA introduction which had the largest aphid populations.
Conclusions
Despite very high levels of GPA being established in the trial, GPA feeding damage to flowering canola did not impact on the growth of the canola plants or on yield. This demonstrates that GPA feeding damage is unlikely to be a major cause of yield loss on its own.
However, GPA can be a vector for viruses and often colonises canola in combination with other aphid species such as cabbage aphid which has been shown to reduce yield and seed quality through feeding damage. This research does highlight that it is important to determine the species of aphid colonising the plant and likelihood of virus infection to manage GPA effectively. The over use of insecticides to control feeding damage of GPA alone may be a waste of insecticide and contribute to the evolution of insecticide resistance.
Further research is needed to determine if GPA colonisation of canola at the seedling stage and subsequent feeding damage causes yield loss in canola.
Acknowledgements
Thanks to Stephanie Boyce for trial management and measurements.
The research undertaken as part of this project is made possible by funding from Royalties for Regions as well as the significant contributions of growers through Grains Research and Development Corporation. The authors would like to thank them for their continued support.
