The importance of soil structure
Soils that have stable aggregates are invariably more productive than soils with unstable aggregates. Stable aggregates allow movement of air and water to plant roots, can usually store more water, and retain their shape when subjected to rainfall and traffic.
Soils that lose aggregate stability through slaking or dispersion have reduced productivity.
Clays and loamy-textured soils are prone to slaking and dispersion and the development of crusts and hardset layers if they have:
- high sodicity: exchangeable sodium >6% of the cation exchange capacity
- low organic matter: <1% soil organic carbon
- low soluble salts: electrical conductivity in 1:5 soil:water, EC1:5 <20 millisiemens per metre (mS/m)
- exchangeable calcium-to-magnesium (Ca:Mg) ratio <2.
Tillage and traffic break down aggregates, and increase the risk and severity of slaking and dispersion in clay and loamy textured soils. Annual cropped soils have more tillage and traffic than annual pastures, and are generally more prone to aggregate instability.
What is cation exchange capacity (CEC)?
Clay minerals in the soil are negatively charged (anions) and adsorb positively charged cations.
The major soil cations are aluminium, Al3+; calcium, Ca2+; magnesium, Mg2+; potassium, K+; and sodium, Na+. The total amount of positive charge that a soil can hold on negatively charged soil particles is expressed as the cation exchange capacity (CEC). CEC is also relatively high on the surfaces of soil organic matter. The higher the CEC, the more able the soil is to hold plant nutrients.
Measuring CEC
Cations are measured by displacing them with a relatively strong solution of uncommon cations such as barium, Ba2+, or ammonium, NH4+. If soils have moderate to high concentration of salts – above 30 mS/m measured in 1:5 soil:water – the cations in soil solution will falsely increase the cations being measured as CEC. To overcome this false reading, the soil sample is washed with aqueous ethanol to remove soluble cations (and anions) leaving only exchangeable cations to be measured.
An approximation of CEC is often derived from the sum of the major base cations: Na+, Ca2+, Mg2+ and K+. They are called base cations because oxides of these metals dissolved in water form hydroxide solutions which are alkaline or basic.
As soils become acidic, hydrogen and aluminium ions in various forms can substitute for some of these base cations, reducing the amount of nutrient cations Ca2+, Mg2+ and K+ in the soil. Base saturation ratio is a measure of the proportion of the soil CEC ‘occupied’ by base cations. When these cations are in solution with other anions (such as sulfate and chloride) balancing the electrical charge, the solution is neutral and has no influence on soil pH.
The importance of cation types
The relative proportions of major cations associated with the clay minerals affects the stability and structure of the soil, if clay is a major contributor to soil texture and structure. Sands have insufficient clay to influence structure. In sands, organic matter is the dominant component contributing to soil CEC, and cation ratios have no influence on sand soil structure.
Of the 4 major cations, there are 2 ratios that can be used as indicators of soil fertility and structural stability.
Exchangeable sodium percentage (ESP)
In many Australian soils, sodium is prevalent both adsorbed on cation exchange sites and in soil solution. The ratio of exchangeable sodium to the total of exchangeable cations – the exchangeable sodium percentage or ESP – is a good indicator of soil structural stability. Soil testing laboratories can test for the common exchangeable cations – Na+, K+, Ca2+, Mg2+, Al3+. The sum of these is the CEC (explained above), and the ESP can be calculated as:
ESP = (exchangeable Na+/CEC) x 100
Sandy loam and heavier topsoils with ESP higher than 6 and subsoils with ESP higher than 16 are deemed sodic and likely to have poor structure due to clay dispersion with associated hardsetting, low porosity and high bulk density leading to poor plant root growth and production.
The calcium-to-magnesium (Ca:Mg) ratio
Worldwide and Australian field work indicates there is a wide range of Ca:Mg ratios over which crop production remains optimum. Poor plant production at extremes of the Ca:Mg ratio can be attributed to either calcium or magnesium deficiency.
How the Ca:Mg ratio and ESP interact
Low Ca:Mg ratios occur when ESP is high (Figure 1). Low Ca:Mg ratios and high ESP can both indicate poor soil structure in clay-dominant soils. However, soil organic matter and dissolved salts (moreso in subsoils) modify aggregate structure.
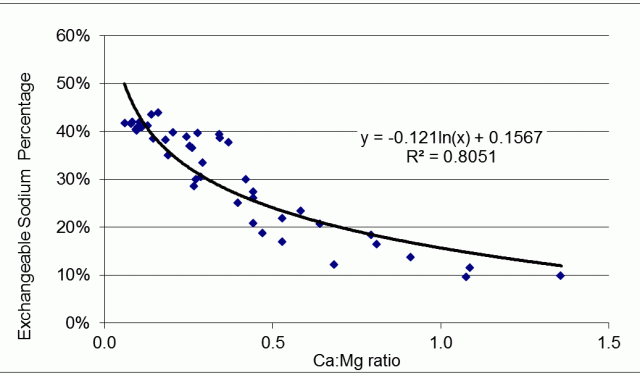
Soils with a high concentration of ions in soil solution tend to be relatively well structured: saline soils are an example. The salinity values that will change dispersion, vary with soil organic matter, the types of salts present, and cultivation. Adding fresh water to saline sodic soils will cause dispersion not evident at lower soil-water content. Sodic surface soils disperse readily when they are wet.
How added gypsum affects ESP and the Ca:Mg ratio
Gypsum is commonly used as a soil amendment to improve structure and drainage of sodic soils.
When gypsum is added to the soil it has 2 effects:
- a transient effect of dissolved gypsum in the soil solution which moves down through the soil profile with water over a few seasons
- the other, more-lasting effect is replacing sodium adsorbed on clay surfaces with calcium. This lowers the ESP and raises the Ca:Mg ratio at the same time. Sodium ions displaced by calcium can be leached deeper in the profile balanced by the sulfate ions from gypsum.
Table 1 and Figure 2 illustrate the changes in cation balance as a result of applying gypsum to the surface of a poorly structured clay soil. Layers down to at least 30 cm were influenced, but there was no effect at 50 cm or deeper.
| Rate of gypsum applied | Ca cmolc+/kg* | Mg cmolc+/kg | K cmolc+/kg | Na cmolc+/kg | Ca:Mg | ESP |
|---|---|---|---|---|---|---|
| Nil | 2.08 | 6.92 | 0.63 | 3.92 | 0.30 | 29 |
| 10t/ha* | 4.06 | 6.08 | 0.53 | 2.61 | 0.67 | 20 |
* cmolc+/kg = centimoles of positive charge per kilogram of soil; t/ha = tonnes per hectare
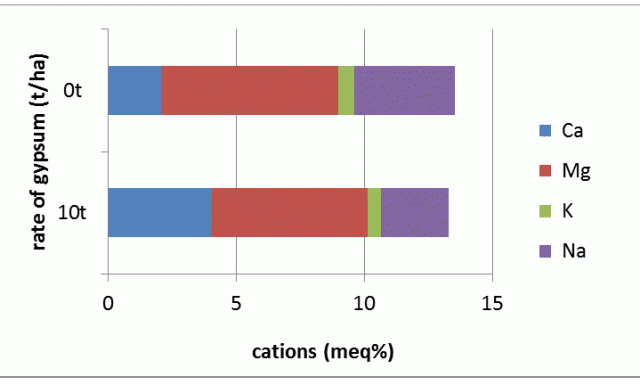
After 20 years, gypsum still replaces about one-third of the adsorbed sodium on the clay. This is measured as a reduction in the amount of sodium and an increase in the amount of calcium on the cation exchange sites and can be expressed as a reduction in the ESP and an increase of the Ca:Mg ratio. There have been small changes in the amounts of exchangeable magnesium and potassium in the upper layers.
The soil in all layers and gypsum rates at this site remains well below the ideal Ca:Mg ratio of 7, yet crops grow successfully and profitably across this site. For the layer presented, the ESP is approaching the critical subsoil value of 16, the ESP of the surface layer at this site was reduced from 17 to 11 after 10 t/ha of gypsum was applied, but remains above the suggested level of 6 for surface soil.

