Equipment needed for a chicken necropsy
- standard post-mortem kit including scalpel, forceps, scissors
- secateurs
- dry swabs, jars for individual fresh samples and pooled formalin fixed tissues
- swabs in media for bacterial culture
- 0.5 millilitre (mL) sterile saline (drip fluid) or viral transport media (VTM).
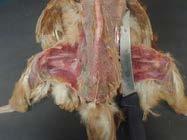
Skin and feathers removed displaying:
Coelomic musculature
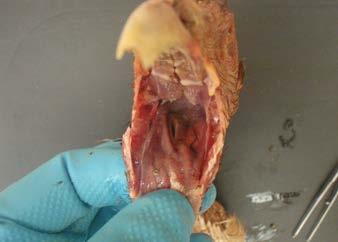
Neck and cranial coelom
Coelomic cavity
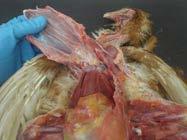
Coelomic cavity with gastrointestinal tract reflected
Gastrointestinal tract anatomy
External examination
Use dry swabs to firmly swab the cloaca and trachea mucosa, then snap swab heads into separate viral transport media (VTM) or saline for avian influenza and Newcastle disease virus testing. (VTM is available from AHL or district veterinary officers.)
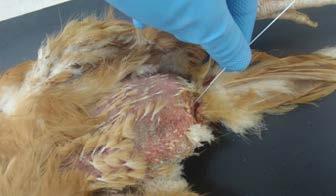
Examine the feathers, skin and limbs. Use sticky tape to collect mites and tape to a microscope slide for identification. Take skin scrapes of crusting lesions to reveal mites.
Collect a tracheal swab
Collect a cloacal swab
Post-mortem approach
1. Pluck the feathers from the dorsum and ventrum
2. Disarticulate the leg
3. Expose the pectoral muscle
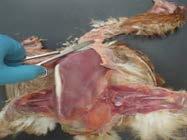
4. Visualise the air sacs while removing keel/pectoral muscle
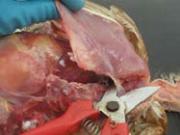
5. Remove the sternum
6. Reflect the coelomic musculature
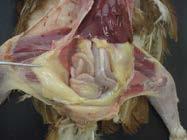
7. Visualise the bursa in dorsal cloacal wall
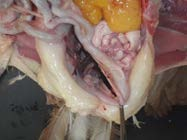
8. Remove the heart
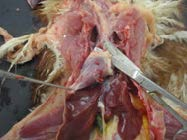
9. Cut the base of the oesphagus
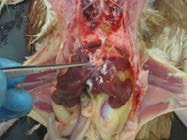
10. Retract the gastrointestinal tract to expose the reproductive tract
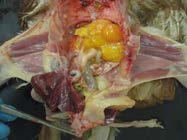
11. Anatomy after removal of the reproductive tract
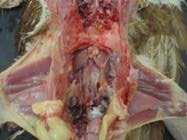
12. Gently peel the lungs from the rib cage
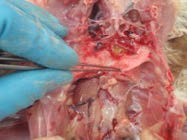
13. Open the crop and oesophagus
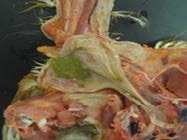
14. Location of larynx

15. Swab the sinuses for exudates
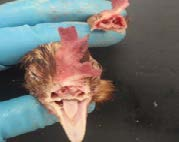
16. Brain exposed for sampling
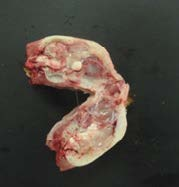
Remember: Sample lesions if not included in the samples mentioned above; fresh and fixed samples are ideal if lesions are large enough.
For more information, please contact the Animal Health Laboratories.