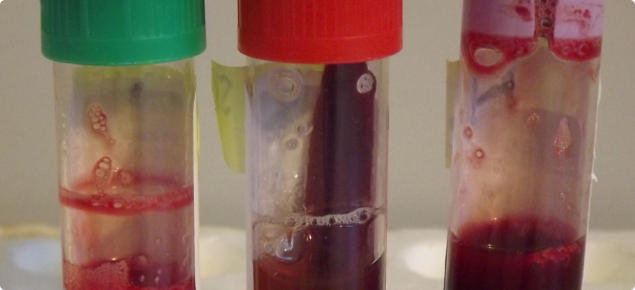
Matching the tube to the blood test
Sample requirements differ depending on whether the test request is for virology, serology, clinical biochemistry and/or haematology. The correct tubes for various applications are listed in Table 1.
| Tube type | Cap colour | Amount in millilitres (mL) | Tests performed |
|---|---|---|---|
| Clotted (plain) |
| 10 |
|
| Lithium heparin |
| 10 |
|
| Ethylenediaminetetraacetic acid (EDTA) |
| 5 |
|
Do not use fluoride oxalate tubes and specialist coagulation profile tubes for the tests in Table 1 above. Where specialised tests are required, contact DDLS on +61 (0)8 9368 3351 before submission.
What makes disease diagnosis difficult?
The following issues can make it difficult to test samples and/or to interpret test results:
Haemolysis
Turbulent collection, rapid temperature changes, moisture, and rough handling increase haemolysis.
Solution
Remove the needle before expressing blood from a syringe into a tube as increased pressure causes haemolysis. Let samples cool to room temperature before refrigeration. Leave plain tubes to clot. To mix anticoagulant tubes, gently tilt from end to end.
Insufficient sample
Incorrect ratios of anticoagulant to blood may affect results. Insufficient volume will also limit the number of tests available.
Solution
Fill blood tubes to the indicated line to ensure correct anticoagulant concentration and to provide sufficient blood for multiple tests.
Too few samples
Solution
Take samples from cohort animals to allow thorough investigation of herd disease status. Ten blood samples are ideal. Supplying multiple samples reduces the risk of misinterpretation due to individual or diurnal variation.
Aged samples
Some blood parameters can break down quickly following collection. Depending on the tests required, collection and receival at the laboratory can be critical for getting an accurate result.
Solution
For haematology and thiamine tests, samples should be received by DDLS within 24 hours of collection. Samples taken for biochemistry tests should be received by DDLS within 48 hours of collection and kept cool (not frozen). Samples collected for virus testing can be received after 48 hours as once clotted, antibodies in serum remain stable for a prolonged period.
Prior treatment
Blood sampling after veterinary treatment can significantly alter results.
Solution
Consider collecting blood before treatment and storing samples pending response to treatment. List any prior treatments on DDLS submission form.
More information
Contact DDLS on +61 (0)8 9368 3351 or see the livestock biosecurity program contacts webpage.