Pre-harvest protection
With harvest fast approaching there is a narrowing window for weed, pest and disease management before the season ends. Crop topping, swathing (with or without an additional spray application) plus crop desiccation or pre-harvest spraying remain useful options for spring management.
ALWAYS check for changes to registrations and rates of applications for any chemical to be applied at this late crop stage. ALSO, grain receival standards need to be adhered to when delivering grain to CBH and other grain marketing organisations.
For information on CBH's receival standards refer to Maximum residue limits three strike approach.
Swathing and spraying canola
While the seed set control of weeds can be an added bonus of swathing and dessication, the timing of these operations to optimise grain yield and oil content of canola can be a challenge.
Glen Riethmuller from the Department of Primary Industries and Regional Development (DPIRD), conducted a number of experiments in Katanning and Mt Barker, from 2010-2013, on the effects of desiccation, swathing and pre-harvest spraying on annual ryegrass seed viability and canola yield and oil parameters. This research was co-funded by GRDC under the Australian Herbicide Resistance Initiative Phase 4 project.
He found that the viability of annual ryegrass seed can be reduced substantially by desiccation, pre-harvest spraying or swathing of canola depending on the product and timing.
In 2013 at Katanning, a pre-harvest spray with greater than 2.0 L/ha glyphosate (Weedmaster® DST®) reduced ryegrass seed viability by an average of 96% while desiccation with 400 g a.i./ha diquat reduced viability by 85% (p<0.05) (Table 1). There was no difference between canola yield or oil content for all of the spray treatments or their times of application. Average canola yield was 1.15 t/ha and average oil content was 49.0%.
| Treatment | Viable ryegrass density* (seed/m2) |
| Nil, no crop top | 14500 a |
| Desiccation 400 g a.i./ha diquat | 2190 bc |
| Desiccation 600 g a.i./ha diquat | 1370 bc |
| Pre-harvest 2L/ha weedmaster® 20% | 256 c |
| Pre-harvest 3L/ha weedmaster® 20% | 282 c |
| Pre-harvest 4L/ha weedmaster® 20% | 572 c |
| Pre-harvest 2L/ha weedmaster® 50% | 833 bc |
| Pre-harvest 3L/ha weedmaster® 50% | 729 bc |
| Pre-harvest 4L/ha weedmaster® 50% | 190 c |
| Average | 1410 |
| Lsd (p<0.05) P-value C of V% | 2768 <0.001 118.8 |
*Means with similar letters in a column are not significantly different at the 5% level.
Desiccation treatments applied in 100L/ha at 80% colour change of canola seed.
Pre-harvest treatments applied in 80L/ha at 20% or 50% colour change of canola seed.

The results across all years (from 2010 to 2013), showed that all treatments such as desiccation with diquat, a pre-harvest spray with glyphosate or spraying glyphosate on a swather can reduce the viability of annual ryegrass. The ability of glyphosate to reduce seed viability when applied pre-harvest however depended on the timing. Applications were targeted at the best stage for the canola crop and not necessarily for the ryegrass so was not always optimum for ryegrass seed management.
The results for diquat were also quite variable. On average (across all application rates), desiccation with diquat reduced the viable ryegrass seed production by 65% in 2010 and 88% in 2013 but did not reduce viability at all in 2011 or 2012. This may also have been due to the spray timing being too late for the ryegrass.
All desiccation and pre-harvest treatments had no effect on canola yield or oil content in 2012 and 2013 at Katanning but there were some small but variable reductions in yield in 2010 and oil in 2011 at Mt Barker.
The canola seed size was reduced with the diquat treatment in 2013.
For the complete details and results from this research, please refer to Glen’s 2017 Research Update paper Annual ryegrass viable seed reduced by dessication and swathing canola.
Crop topping
Crop topping (the late application of herbicides to prevent weed seed-set) can be used for managing herbicide resistance, as a late post-emergent salvage treatment or to control any weeds that survived or were missed in other weed management strategies. Late weed control may also reduce grain contamination but a crop-topping application will not increase grain yield.
There are paraquat and glyphosate products (please read the label) registered for crop-topping in WA but these are limited for use in pulse crops and predominantly target annual ryegrass.
For further information on best timing for weed management while limiting potential impacts on the crop refer to Crop topping pulse crops.
Pulse Australia Glyphosate (lupins) permit 81595.
Timing of wild radish for in-crop seedset control
The embryo is the key to controlling seedset in wild radish. Research conducted by Dr Aik Cheam (previously DPIRD) has found that the highest level (up to 100 per cent) of seedset control is achieved when control measures are applied after flowering and in the 21 days before the embryo is formed.
Use the rating system below to decide what stage your wild radish is in, so that you can achieve the greatest efficacy. Assessment of the growth stages should take place a few days after flowering.
- Remove a few seed pods from at least 10 plants
- Split open the pods and check for embryo development within the seeds
- Repeat this procedure every few days
- Make assessments according to the rating system (see Table 2)
| Timing | Description of growth stage |
| Pre-embryo The pre-embryo stages last about 21 days from the time of flowering | Stage 1 Early flowering and newly formed thin pods present. |
| Stage 2 Mid-flowering and pod fill; well-formed pods are still squashy and watery when pressed between the finger and thumb. Seed development at ovule stage with no embryo (green fleshy interior). Squeeze between thumb and finger to obtain a globule of seed which is a mass of cells with no embryo. Growers can carry out this procedure in the paddock. | |
| Post-embryo The embryo forms about 21 days after flowering | Stage 3 Embryo formed; pods still squashy and watery but newly-formed embryo already present. |
| Stage 4 Late flowering and pod development; pods turned woody. Green, well-developed embryos present when pods are crushed. |
Slashing, mowing, hand-weeding, green and brown manuring
In the pre-embryo stage, slashing, mowing, hand-weeding and green and brown manuring result in up to 100 per cent seed reduction. In a cropping situation, this will sacrifice a poor crop but it is a valid means of reducing wild radish seed numbers.
Selective spraytopping
In trials over several years, spraytopping applied pre-embryo provided good seedset control of wild radish in lupins that have stopped flowering. It is not recommended for field peas.
Croptopping with dessicants
Not recommended in lupins or any pulse crop for wild radish seedset control. By the time the crop reaches physiological maturity, a high proportion of the wild radish seeds in the crop have reached the embryo stage and the application of desiccants is futile.
Weed wiping
Weed wiping selectively applies herbicide by brushing it directly on the weed and relies on the weed being at least 20 cm taller than the crop canopy. Due to this physical separation, desiccants or translocated herbicides can be applied at the pre-embryo wild radish stage to achieve good control. The contact herbicide paraquat is generally less effective than glyphosate because the treated wild radish is likely to recover and set seed.
Ergot
Ergot is a fungal disease of grasses and cereals which produces toxic fruiting bodies that replace the seed. Almost all ergot in grain in Western Australia comes from ryegrass that grows within a crop. The ergots are black, elongated in shape, and measure 2mm to greater than 20 mm.
For more information refer to Diagnosing ergot.
Control of ergot in crops with pre-harvest desiccants
Growers should be aware that ergot specifications in barley are decreasing; in the 2017/18 harvest specifications, ergot up to 30mm in size will be accepted but in 2018/19 the size limit will be reduced to 20mm where it will remain.
Grassy areas of crop with a paddock history of ergot may benefit from a late season herbicide application to kill ryegrass to prevent ergots forming and contaminating grain. The alternative is to clean grain prior to delivery. Table 3 shows the late season options for wheat and barley.
In 2016 the APVMA granted a permit for glyphosate use in feed or food barley; it remains illegal to apply glyphosate and deliver the grain into a malting stack. Growers are advised not to apply glyphosate to seed crops as germination can be compromised. Only some glyphosate products are covered under the permit and the onus is on growers to check for current registrations and to obey harvest withholding periods and other guidelines.
| Crop type | Paraquat | Diquat | Glyphosate |
| Wheat | no | yes | yes |
| Barley - malting | no | yes | no |
| Barley - feed/food | no | yes | yes |
For more information refer to GRDC's Pre-harvest herbicide use fact sheet. Please note, that this fact sheet was last revised in October 2014 so some registrations may have been altered.
Green radish pods can reduce crop seed viability in storage
Grain harvested for seed must either be free from green wild radish pods when harvested or cleaned immediately after harvest.
Work by Dr Aik Cheam, formerly from DAFWA (now retired), showed that green radish pods harvested and stored with grain can reduce the viability the grain within a few days of storage. The gases emitted from the green radish pod, as it desiccates, are toxic to wheat, barley, lupin and field pea seed (and possibly other crops although this is untested).
The impact on seed viability was dependent on:
- the level of radish contamination,
- the storage temperature (the higher the temperature the greater the impact)
- the length of time the seed is stored with the radish contaminant.
Results from one experiment indicated that crop seed germination was affected when green radish pods, at a 5% contamination level, were stored with seed for three days after harvest at field temperatures.
- At a 10% contamination, the viability of all lupin seed was lost after 5 days of storage.
- Field peas, although slightly less sensitive than lupins, still showed a 98 to 99 % loss of germination when contaminated with 10% radish for one week.
- For cereals, a 5% contamination level in wheat and barley (stored for one week) reduced seed viability by 40 to 100%, with wheat varieties being more sensitive.
Several actions are worth considering if green wild radish is found growing in crops at harvest time.
- Minimise the impact, and avoid retaining seed from areas with high densities of green radish.
- If green wild radish is harvested with the seed, grade to remove the radish pod within hours of harvesting. (If you are stuck, harvest on a cool day and store the grain on the floor of the super shed until cleaning).
- Swath the crop to allow the wild radish to dry out in the windrow before harvesting.
- Burn off the green wild radish with a desiccant such as diquat. Please check the label before application.
- Always check registrations, more information can be found in the GRDC Factsheet – Pre-Harvest herbicide use. Please note, that this fact sheet was last revised in October 2014 so some registrations may have been altered.
Further studies by Aik Cheam found that storing seed from plants that had been treated with diquat prior to harvest (such as a pre-harvest desiccation application) showed that there was no impact of dry radish seed on lupin seed viability.
Insect grain contaminants
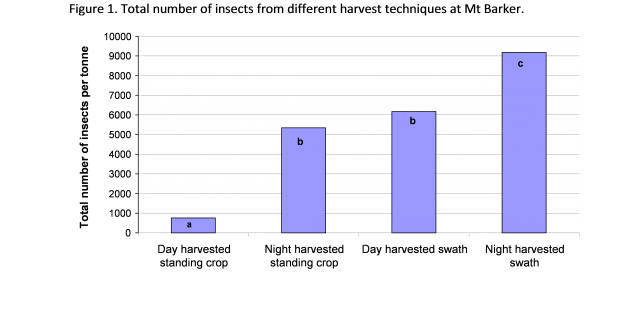
There are almost no options for management of insect pests pre-harvest. However there are ways that contamination of harvested grain by insects can be minimised. Research done by Svet Micic (DPIRD) in 2004 (co-investment with GRDC) investigated the effect of harvest time and type of harvest (standing crop vs swath) on the subsequent level of insects and molluscs in the grain.
Time of harvest can affect numbers of vagrant insects i.e. those insects incidentally harvested with the grain. For direct harvested crops, harvesting during the hottest part of the day can lead to a reduction in the numbers of European earwigs, snails and beetles being incidentally harvested. These insects tend to move at night and higher numbers can be found in grain harvested during the night than the day (See Figure 1 below).
If harvesting swaths, consider harvesting as soon as practicable. The longer a swath is on the ground the more vagrant insects can be found harbouring under or in the swath and the more are incidentally harvested. Also consider swath height,. Swaths that are close to or on the ground tend to have more vagrant insects in the grain.
Key messages from this research are:
- Direct harvested grain contains significantly less insect contaminants than grains harvested from swaths.
- Grain harvested at night contains more insect contaminants than grain from crops harvested during the day.
- Harvester fronts do not influence the abundance of insect contaminants in grain.
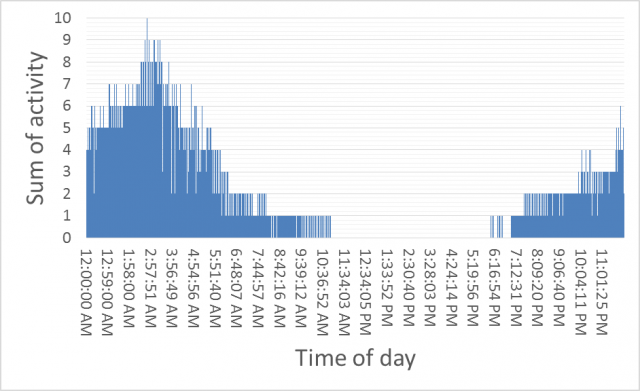
As a part of the Boosting Grains Research and Development Flagship supported by Royalties for Regions, research is being conducted to determine when the best time of day to harvest grain is to minimize contamination by small pointed (conical) snails. From October 2016 until February 2017 remote cameras were used to monitor the movement of snails up and down stalks of barley. The rate of movement was scored. Most snail movement was linked to time of day with no snails moving from 11 am until 6 pm (Figure 2). Thus harvesting during the day will lead to less snails being found in harvested grain.
Early control of summer weeds
Control of summer weeds is best done when the weeds are small – particularly those weeds that may be difficult to manage as they age or when growing conditions are challenging (hot, dry and dusty).
Many of our summer weeds, like Fleabane, will germinate in late spring following rains at harvest. Take the time during the harvest operation to identify paddocks and other areas where summer weeds are germinating and then return to these paddocks following harvest to control the seedlings.
For more information on summer weeds and their control see the Summer weeds webpage.
Contacts
Swathing and spraying canola
Glen Riethmuller
Development Officer
glen.riethmuller@dpird.wa.gov.au
Ph: +61 (0)8 9081 3146
Weeds
Alex Douglas
Research Officer
alex.douglas@dpird.wa.gov.au
Ph: +61 (0)8 9821 3246
Insects
Svetlana Micic
Entomologist
svetlana.micic@dpird.wa.gov.au
m: 0427 772 051