Nitrate leaching
Ammonium-based fertilisers are major contributors to soil acidification.
Nitrogen in agricultural systems may be fixed from the atmosphere by legumes, decomposed from soil organic matter (the dead remains of plants and animals) by soil organisms, or added in various types of fertilisers. Different nitrogen fertilisers follow slightly different chemical pathways as they break down in the soil and contribute different amounts of hydrogen ions (acid) to the soil.
Fertiliser nitrogen that enters and leaves the system in the same form does not contribute to soil acidification, for example, potassium nitrate. Nitrogen that stays in the system does not contribute to soil acidification, for example, nitrogen added to the soil as fertiliser is taken up by a plant and then returned to the soil when the plant dies and decomposes and is taken up by another plant and so on (Figure 1).
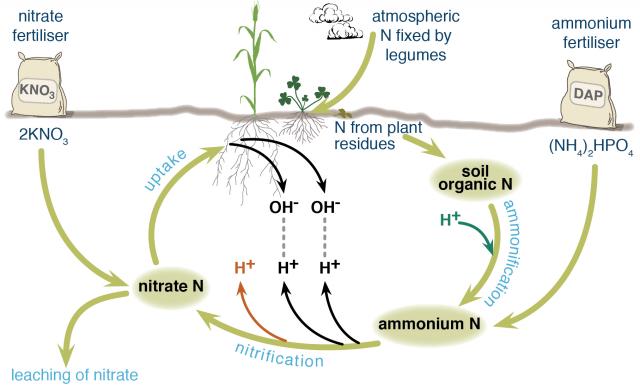
Ammonium-based fertilisers are the major contributors to soil acidification, especially if the nitrogen is leached rather than taken up by plants. Only if the nitrogen is returned to the soil again when the plant dies is there no acidification. Many ammonium fertilisers contribute to soil acidification even if the nitrogen is taken up by plants.
Ammonium nitrogen from fertiliser or soil organic matter is readily converted to nitrate and hydrogen ions by bacteria in the soil. This contributes different amounts of hydrogen ions to the soil, depending on the fertiliser.
When nitrate, which is negatively charged, is taken up by plants, a hydroxide ion, also negatively charged, is released from the plant to maintain electrical balance. This hydroxide ion combines with a hydrogen ion in the soil to form water (the hydrogen ion is no longer contributing to soil acidity). Depending on the fertiliser, all hydrogen ions released by nitrification may be neutralised or there may be a net increase in hydrogen ions.
If nitrate is not taken up by plants, it can leach away from the root zone, meaning that no hydroxide ion is released from the plant to bind with a hydrogen ion. Nitrate ions are readily leached from most Western Australian agricultural soils because there are more negatively charged surface sites on soil constituents than positively charged surface sites (required to retain the negatively charged nitrate ions). If nitrate leaches, a positively charged cation is also leached to maintain electrical balance.
The cations that leach are usually sodium, potassium or calcium rather than hydrogen, because hydrogen ions are more strongly held by the soil. If the nitrate is from ammonium fertiliser, the result is a net increase in hydrogen ions.
Figure shows how ammonium and nitrate fertilisers enter the nitrogen cycle in different places. Nitrification by bacteria of one molecule of diammonium phosphate (DAP) fertiliser would release three hydrogen ions and two nitrate ions into the soil.
If the two nitrate ions were taken up by a plant, two of the hydrogen ions would bind with hydroxide ions released from the plant, leaving one hydrogen ion contributing to soil acidity. If the two nitrate ions leached away from the root zone, all three hydrogen ions would remain to contribute to soil acidity.
If nitrate ions taken up by the plant are from potassium nitrate fertiliser, there is a liming effect because hydrogen ions are neutralised in the process. If those nitrate ions are leached, there is no liming effect, but also no soil acidification because no hydrogen ions are contributed to the soil with the fertiliser.

