Soil acidity messages for farmers from Greener Pastures:
- Soil test all paddocks to obtain pH values to assess if soil acidification is likely to be reducing pasture production.
- If soil acidification is identified as a problem, start liming with as large levels of good quality lime as can be afforded until the desired pH is achieved.
- Soil acidification is ameliorated by applying sufficient good quality lime to raise the pHCa of the top 10cm of soil to 5.5 or greater.
- Where possible, lime should be incorporated into soil after application to increase its effectiveness.
- Soil acidification continues, both while liming and after liming has been successfully completed, particularly for intensively grazed pastures top-dressed with fertiliser nitrogen after each grazing.
- Once the target pH has been achieved, monitor soil pH and re-apply lime when pHCa declines below 5.5, requiring smaller amounts of lime (1t/ha) to return soil pHCa to 5.5 or greater.
- Do not allow soil pHCa to decline much below 5.5 to avoid a major re-liming program to ameliorate the problem.
What did we learn about soil acidity in Greener Pastures?
Soil sampling at Vasse Research Centre
During 1999-2009, soil testing for pH was used to determine lime application for 48 paddocks at the Vasse Research Centre at Busselton, in the south-west of Western Australia (WA).
Paddocks had been grazed intensively by dairy cows and their young stock over a period of 10 years, as part of the Vasse Milk Farmlets and Greener Pastures farming system projects.
Pasture consisted of annual ryegrasses with some subterranean clover. Soils in the 48 paddocks were 1–2m sand to sandy loam over massive clay, known locally as Abba sand. For many soils in the region, including Abba sands, the topography is flat and the soils are waterlogged from June to early September in the typical May to November growing season.
No major liming program had been undertaken in the 48 paddocks before April 1999 and soil testing in 1999 indicated soil pHCa for the top 10cm of soil was 4.0–5.0 in all paddocks.
Soil acidification was therefore identified as a major problem, and a liming program was undertaken to rectify the problem, starting in 1999.
The soil sampling program
Samples of the top 10cm of soil were collected from each paddock in April 1999 and January–February 2000–2009, during the dry period before fertiliser was applied. These are the standard sampling depth and sampling time for soil sampling of dryland pastures in WA.
Soil samples were collected while walking on the same diagonal path across each paddock each year between two permanent markers located on fences. Samples were collected using 2.5cm diameter metal tubes (10cm long; known locally as pogos) that were pushed into the soil by foot every 2–3m, with 50–100 samples collected per paddock, depending on the size of the paddock.
The samples from each paddock were bulked, air dried and sieved through a 2mm sieve to exclude coarse material. The samples were sent for analysis to CSBP Laboratories in Perth, WA. Soil pH was measured by mixing soil and 0.01 molar calcium chloride in the ratio of 1 weight of soil to 5 volumes of calcium chloride, which is the standard procedure for measuring soil pH in Australia and much of the world.
Liming the 48 paddocks
Local ground limestone with a neutralising value of 80–90% was used to ameliorate soil acidification in the 48 paddocks. The lime was spread over the soil surface in March-May, before the start of the growing season, and was not incorporated into soil after application. Finance limited the amount of lime purchased and applied each year and no lime was applied in 2001, 2002 and 2005.
The same amount of lime was applied to all 48 paddocks in 1999 (3t/ha), 2000 (4t/ha) and 2003 (2t/ha). In 2004, 17 of the 48 paddocks with the lowest soil pH were treated with 1t/ha lime.
Lime was applied each year during 2006-2009 and the level of lime applied varied with soil pH. The pH levels that determined lime quantity are shown in Table 1.
| Year | Below 4.5 | 4.9 | 5.0-6.0 | Above 6.0 |
|---|---|---|---|---|
| 2006 | 5 | 2.5 | 1 | 0 |
| 2007 | 5 | 3 | 2 | 0 |
| Year | Below 5.0 | 5.0-5.4 | 5.5-6.0 | Above 6.0 |
| 2008 | 5 | 3 | 1 | 0 |
| 2009 | 5 | 3 | 1 | 0 |
What happened to soil pH?
Liming increased soil pHCa from 4.0–5.0 in 1999 to 5.2–6.0 in 2009 (Figure 1).
Soil pHCa of 5.5 was achieved in individual paddocks by 2007–2009, 9–11 years after the liming program started, and required a total of 12–21t/ha lime for individual paddocks.
For the last soil testing in 2009, 14 paddocks (about 29% of paddocks) were yet to achieve pHCa 5.5, but pHCa values for these paddocks were 5.2– 5.4, so close to the target value.
Measuring soil pH identified soil acidification as a problem in all 48 paddocks and soil testing for pH each year enabled the progress of liming to be monitored.
false
Progress in ameliorating soil acidification was disappointing during 1999-2003 (Figure 1). There were three possible reasons for this:
- Lime was not incorporated after application to the soil surface so liming was less effective.
- Continued soil acidification during 1999–2009 increased the requirement of lime to ameliorate the acidity.
- Amounts of lime applied were limited by finance, so no lime was applied in some years and only low levels of lime were applied in other years.
As the liming program continued and liming eventually increased soil pH, applications of lime after 2006 were more effective at ameliorating the problem (Figure 1).
Though lime is more effective at ameliorating soil acidification when it is incorporated after application, farmers are often reluctant to cultivate soils because, in many situations, the topography is flat and the soils are waterlogged for most of the growing season. After cultivation, managing wet, unconsolidated soils can be difficult. In addition, cultivating soil increases mineralisation of soil organic matter, resulting in loss of soil fertility built up over many years when the soils were not cultivated.
Soil acidity and high rainfall pastures
Causes of soil acidification
Conventional agriculture acidifies soil. As soil becomes more acid, productive pasture species disappear and are replaced by species of low agricultural value. It is a waste of money to apply expensive fertiliser to pastures which are inherently poorly productive because they are growing in an acid soil. Soil acidification takes place via two major processes:
Product removal
Plants take up nutrients from soil solution as either positively charged cations (ammonium, sodium, potassium, calcium, magnesium) or as negatively charged anions (nitrate, chloride, phosphate, sulphate, borate, molybdate) and they usually take up more cations than anions. To maintain internal electrical neutrality, roots secrete hydrogen ions to balance the charge of the extra cations taken from soil.
These hydrogen ions are produced from water in the roots dissociating into positive hydrogen ions (acidic) and negative hydroxide ions (alkaline). This makes the adjacent soil more acid and the plant more alkaline, this is the source of ash alkalinity when plant residues are burnt. A diagrammatic presentation is given in Figure 2.
false
The positive charge of the cations now in the roots is balanced by the negative hydroxide ions remaining in roots. The positive charge of hydrogen ions excreted from roots balances the negative charge of anions left in the soil when the extra cations are taken up.
If no product is removed from the paddock, there is no net loss of nutrients and acidification is negligible as plants break down to become soil organic matter. However, in productive agriculture, plants are removed as produce such as grain and fodder, or as animal products such as milk, meat, wool or hides. This acidifies soil.
The nitrogen cycle
Plants and soil organisms — insects, earthworms, algae, fungi, protozoa and bacteria — break down and become part of the soil organic matter. Grazing animals also return organic matter to soil as faeces and urine.
Soil organic matter is a source of protein, energy and nutrients for plants and soil organisms.
They process soil organic matter by physical and chemical means to release some of its nutrients into soil solution, a process known as mineralisation. Urea in urine patches is a source of nitrogen and potassium for both plants and soil organisms.
Plants take up nitrogen from soil as either ammonium or nitrate. Ammonium is among the first forms of nitrogen mineralised from soil organic matter. Ammonium is also added to soil as fertiliser (ammonium phosphates such as MAP and DAP or ammonium sulphate).
Urea, applied as fertiliser or in urine patches, is rapidly converted to ammonium. Ammonium is positively charged and soil constituents — organic matter, clays, oxides — possess both positive and negative surface charge sites. For most soils, the magnitude of the negative charge is much greater than that of the positive charge. Consequently, ammonium is usually retained by negative charge sites and is not usually leached deeper into soil. In very sandy soils, with insufficient negative charge to retain all the ammonium mineralised from organic matter or applied as fertiliser, some can be leached.
In addition, the amount of ammonium and potassium present in urine patches can greatly exceed the capacity of soil to retain these elements, so both can be leached from urine patches. A diagrammatic presentation is given in Figure 3.
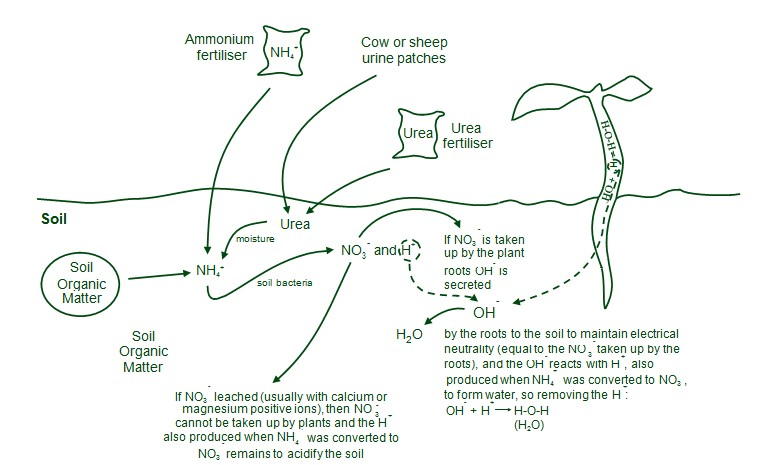
When plant roots take up nitrogen from soil as positive ammonium ions, positive hydrogen ions produced from water in the roots are excreted to maintain electrical neutrality. This acidifies the soil. The hydroxide ions also produced remain in the plant and make it more alkaline — another source of ash alkalinity when plant residues are burnt.
Soil bacteria convert ammonium to nitrate and hydrogen. When plant roots take up nitrogen as negative nitrate ions, hydroxide ions derived from water in roots are secreted into the soil to maintain electrical neutrality. The hydrogen ions also produced remain in the plant and make it more acid. When hydroxide ions are released into the soil, they react with the hydrogen ions to produce water.
Therefore, when ammonium ions are converted to nitrate and hydrogen ions, provided all the nitrate ions are taken up by plant roots, the hydroxide ions secreted in the uptake process consume the hydrogen ions and there is no net acidification of the soil.
However, sandy soils typically have negligible positive charge sites so nitrate ions are readily leached below plant rooting depth. When nitrate is leached, the hydrogen ions remain in the soil and acidify it. Because plants can only take up nitrogen as ammonium or nitrate ions, and ammonium ions are rapidly converted to nitrate ions, leaching of nitrate is a major cause of soil acidification.
Why is soil acidification a problem?
The increased concentration of hydrogen ions in soil resulting from soil acidification:
- increases the solubility (dissolution) of any aluminium and manganese in soil; this can induce manganese toxicity in plants and aluminium toxicity can affect growth and function of plant roots
- increases sorption (binding) of molybdenum by soil, inducing deficiency in plants
- is toxic to the Rhizobia bacteria required for symbiotic nitrogen fixation in legumes.
Aluminium toxicity
Aluminium is a component of clays and oxides in soil, and aluminium compounds are present on the surfaces of many soil constituents. At soil pHCa greater than about 5.0, most aluminium is present in sparingly soluble form so there is negligible soluble aluminium in soil solution. However, as soil pHCa drops below 5.0, there is increased solubility of aluminium.
Aluminium has no known role in plants but, when its concentration in soil solution increases, it becomes toxic to plant roots. Aluminium toxicity greatly reduces root growth and function, reducing the ability of the roots to grow in soil and take up water and nutrients. Aluminium toxicity is the major soil acidity problem for agriculture in WA.
Manganese toxicity
In most agricultural areas, manganese toxicity is usually the first problem resulting from soil acidification. Below about pHCa 5.5, solubility of manganese increases as the concentration of hydrogen ions increases. Plant roots take up soluble manganese from the soil solution. As the concentration of manganese increases, plants eventually take up toxic amounts, reducing yield. Fortunately, the amount of manganese present in most agricultural soils in WA is low. Though solubility of manganese continues to increase as soils acidify, the amount of manganese dissolved is insufficient to cause toxicity for pastures.
Testing for exchangeable soil aluminium
Exchangeable soil aluminium is the best measure we have for estimating the potential for aluminium toxicity. The cation exchange capacity of a soil is a measure of the amount of negative charge sites on the soil surface and is estimated by measuring the amount of cations balancing the negative sites.
Cation exchange sites are typically balanced by the major soil cations calcium, magnesium, potassium, sodium and, for acidified soils, hydrogen and aluminium.
The amount of aluminium balancing negative charge, called exchangeable aluminium, is used as an indicator of when aluminium toxicity is likely to reduce plant yield.
The proportion of the major cations that balance the total cation exchange capacity of most productive soils is usually 65 to 80% calcium, 10 to 20% magnesium, 3 to 8% potassium, less than 4% sodium and less than 5% aluminium. If the proportion of aluminium on the soil exchange sites rises to over 30%, aluminium toxicity is highly likely to affect production of even the most tolerant crop and pasture species.
Tolerance of subclover and ryegrass to aluminium toxicity
Subclover and annual and Italian ryegrasses, the major pasture species in WA high rainfall pastures, are all relatively tolerant of aluminium toxicity. Aluminium toxicity becomes a major problem for these species when the soil pHCa falls below 4.3. For more sensitive species, such as lucerne and annual medics, aluminium toxicity becomes a major problem when the soil pHCa falls below 5.0.
Molybdenum deficiency and molybdenosis
Plants require very small amounts of molybdenum and soils with pHCa values greater than 5.0 usually have enough molybdenum for pasture production. Very little molybdenum is sorbed (bound) by soil when the pHCa is greater than 5.0. However, as the pHCa of a soil drops below 5.0, its capacity to sorb molybdenum rises, inducing deficiency in pastures.
When land was newly cleared, molybdenum deficiency only occurred on naturally acidic soils and molybdenum fertiliser was applied. However, if too much molybdenum fertiliser is applied to pastures grazed by ruminants, the animals can develop molybdenosis.
Molybdenosis results from molybdenum reacting with sulphur and copper in the rumen to form a poorly soluble compound. This prevents the animals from taking up sufficient copper from their gut and they become copper deficient. When this happens, animals need to be injected with copper supplements. Also, the animals should be grazed on pastures that are lower in molybdenum.
Molybdenum deficiency is best ameliorated by applying sufficient lime to raise soil pHCa to greater than 5.0. It is nearly always better to lime a soil than apply molybdenum fertiliser.
Effect of soil pH on nitrogen fixation
The major pasture species for high rainfall pastures in WA are the annual species annual ryegrass (Lolium rigidum), Italian ryegrass (Lolium multiflorum) and subterranean clover (Trifolium subterraneum). Subterranean clover is the only one of these three pasture species capable of biological nitrogen fixation.
Biological nitrogen fixation is brought about by Rhizobia bacteria in the soil which ‘infect’ the roots of emerging subclover plants to form nodules. In these nodules, the bacteria convert atmospheric nitrogen to nitrogen compounds required by the clover — mainly amino acids and proteins. The dead remains of subclover plants — roots, stems, leaves, burrs and seed — are processed by soil organisms to release nitrogen to the soil, initially as ammonium ions. These are then converted by soil bacteria to nitrate and hydrogen ions, as previously discussed, to provide nitrogen for ryegrass.
The Rhizobia bacterium which infects subclover (Rhizobia trifolii) is more sensitive to low soil pH than the host plant; when the soil pHCa falls below about 4.5, the bacterial population declines. Eventually, nitrogen fixation cannot occur and clover has to obtain nitrogen from the soil. If there is insufficient soil nitrogen, nitrogen deficiency reduces clover and ryegrass production and persistence. Though fertiliser nitrogen can be applied to overcome nitrogen deficiency, aluminium toxicity greatly reduces the ability of roots to take up water and nutrients from the soil.
Measuring soil acidity
Soil pH is the most widely used and simplest method for assessing soil acidification and when to apply lime.
The pH of a solution is the negative logarithm of its hydrogen ion concentration. As the concentration of hydrogen ions increases, pH values decline.
Soil pH was traditionally measured in a suspension of 1 weight of soil to 5 volumes of water. However, the salt content of the soil has an influence on soil pH measured by this method. If salt is added to a soil-water suspension, the pH decreases. To overcome this problem, a salt solution of 0.01 molar calcium chloride in now used instead of water. All pH values quoted in this Bulletin were measured in calcium chloride — pHCa.
The standard depth used in WA when sampling for soil nutrients and acidity is 10cm. If subsurface acidity is considered important, samples should also be collected from 10–20cm and from 30–40cm.
Measuring aluminium concentrations when measuring soil pH
When commercial soil testing laboratories in WA measure soil pH in calcium chloride they will, if requested, also measure the concentration of soluble aluminium present in the extract solution used to measure the pH.
Aluminium toxicity is likely when the concentration of aluminium measured by this procedure is greater than 8–10mg/kg soil. This is not a measure of exchangeable aluminium in soil, which requires a more sophisticated procedure. It is a relatively crude test but allows paddocks with the largest aluminium concentration measured in the extract solution to be given top priority for liming.
Subsoil acidity
Research in the cropping areas of south-western Australia has shown that the decline in soil pH since the start of agriculture is often greater in the subsoil than in surface soil, with the greatest decline from about 10–40cm depth. Extensive research in eastern Australia has shown that subsoil acidity is a major problem for high rainfall pastures in that region.
Until the mid-1990s, there was little evidence for subsoil acidity in the high rainfall areas of WA, probably because these areas were developed much more recently than the high rainfall pastures in eastern Australia. However, since the early 2000s, there is increasing local evidence for subsoil acidity in high rainfall pastures, particularly where surface soil pHCa has been allowed to decline to 4.0 or less.
Whether or not a low subsoil pH reduces the productivity of our generally shallow-rooted pastures has yet to be determined. Subsoil acidity is more difficult and costly to ameliorate than topsoil acidity.
Ameliorating soil acidity
The simplest way to ameliorate soil acidification is to add lime to the soil. When lime is added to moist soil, alkali dissolves from the lime and consumes hydrogen ions. The lime can be left on the soil surface, incorporated into the surface 2cm using heavy harrows or disced into the surface 10cm. Generally speaking, the lower the surface pH, the more necessary incorporation becomes.
Ameliorating subsoil acidity
If the pHCa in the 10–20cm and 20–30cm zones is less than 5.0, subsoil acidity may be a problem. The only practical way to raise the subsoil pH is to apply sufficient lime to raise the pHCa of the top 10cm of soil to 5.5 or greater. When the pHCa of the top soil is above 5.5, the alkali dissolved from the lime moves rapidly into the subsoil. If the subsoil soil pHCa is maintained above about 5.5, subsoil acidity should not be an issue.
Sources of lime
There are three sources of agricultural lime in WA:
Limestone
Limestone (calcium carbonate) is the most common lime source south of Perth; it is found naturally as rock and needs to be crushed to be effective.
Limesand
Limesand (calcium carbonate) is mostly found north of Perth and occurs as sand dune deposits. Unlike limestone, limesand is not usually ground before application because much of it exists as fine, porous, sand-grain sized material.
Dolomite
Dolomite is a mixture of calcium and magnesium carbonates, with most deposits usually comprising about 60% calcium carbonate and 40% magnesium carbonate. It occurs naturally near salt lakes in the cropping areas of the south-west. Dolomite often contains large rocks so is usually ground before application. Because dolomite deposits are usually a long way from high rainfall areas, high transport costs result in dolomite being more expensive than limestone or limesand.
Although magnesium deficiency has not been detected in pastures in WA, dolomite is often recommended to increase soil magnesium levels. To ensure magnesium deficiency does not occur in animals, they can often be supplemented directly.
Lime quality
Lime quality is now evaluated by effective neutralising value (ENV).
ENV is a lime quality testing and reporting procedure that has replaced neutralising value (NV) and particle size previously used. It is a measure of the ability of a lime source to neutralise (ameliorate) soil acidity. In the past, NV was considered the major factor that determined the ability of a lime to reduce soil acidity. Recent research has shown that over 70% of the effectiveness of a lime product in ameliorating soil acidity can be explained by particle size, whereas less than 10% can be explained by NV.
ENV considers four factors: NV, particle size and two other factors, which will be explained below. The NV of a lime source is compared to the NV of pure calcium carbonate which is defined as 100%. If a lime source has a NV value of 80%, then it is 80% as effective as pure calcium carbonate at reducing soil acidity.
Most lime sources in WA have NV values of 60 to 90%, with the best deposits having NV values of 90% or more.
A lime source with fine particles has a greater surface area exposed to the acid (hydrogen ions) in the soil and will neutralise soil acidity faster than coarser lime particles. The carbonate component of lime exposed at the surface of lime particles reacts with hydrogen ions in soil solution or on the surfaces of soil constituents.
The greater the number of carbonate ions exposed at the surface of lime particles, achieved by using finer lime, the quicker soil pH is increased.
The third measure contributing to ENV is a measure of the change in soil pH due to the lime source. This is a laboratory test carried out by mixing a given amount of lime with a given amount of soil, adding water to the mixture and measuring soil pH after the moist mixture has been incubated for a set length of time.
In another sample of the same soil, the same procedure is repeated but no lime is applied. From the two samples, the change in soil pH due to adding lime to the soil can be calculated.
The fourth measure contributing to ENV is a measure of the solubility of the different size fractions of the lime source. To measure lime solubility, the lime sample is separated by sieving into five fractions: 0 to 0.125mm, 0.125 to 0.25mm, 0.25 to 0. 5mm, 0.5 to 1.0mm and greater than 1mm. The percentage of particles that pass through each sieve is recorded. The solubility of lime in the different sieved fractions is then determined.
The combination of all four factors determines the ENV which is expressed as a percentage. The higher the ENV value, the more effective is the lime source at increasing soil pH.
How much lime to apply
There is no simple answer to the question of how much lime is required to achieve a pHCa of 5.5 or greater in the top 10cm of soil. The amount depends on many factors, particularly on how low the pH is currently. To cope with these varying factors, general recommendations are given in Table 1 for the typical amount of lime to apply to raise soil pH.
Amelioration of soil acidity depends on a chemical reaction between the lime and the soil. Because of this, the properties of both soil and lime determine the amount of lime required.
Solubility of lime takes place in moist soil, so rainfall is important. Other climatic factors, such as temperature and humidity, also influence the reaction between soil and lime. Factors that determine the amount of lime required include:
- The soil pH: for any given soil and lime source, more lime is required to raise soil pH
- The lower the soil pH is to start with.
- The pH buffering capacity of the soil, which is the capacity of the soil to retain hydrogen ions. This in turn is largely determined by the amount of clay, iron and aluminium oxides, and organic matter in the soil. The larger the pH buffering capacity, the more lime is required to increase soil pH.
- The bulk density of the soil (mass of soil per unit volume). Soils with more pore space relative to mass of soil particles have lower bulk densities and so need less lime, while soils with larger bulk densities have larger mass of soil per unit volume and so require greater amounts of lime.
- The amount of lateritic ironstone gravel stones, which are particles of greater than 2mm diameter, in the soil. Gravel particles have little impact on soil chemical properties but do influence soil mass and bulk density. Many sandy gravel soils in WA contain more than 60% gravel. These relatively chemically inert gravel stones decrease the amount of lime required per volume of soil to raise soil pH.
- ENV determines the ability of the lime source to increase soil pH. ENV depends on the NV and particle size of the lime source used, how soluble different size fractions of the lime are in the soil and the magnitude of the increase in soil pH due to adding the lime source to the soil.
- Soil moisture. The speed at which lime dissolves in the soil to consume hydrogen ions, and the speed at which it moves down the soil to ameliorate subsoil acidity, depends on soil moisture, including rainfall, soil temperature and humidity. The longer the lime is in moist, warm soil, the more rapidly the lime reduces soil acidity.
- Tillage. The extent of mixing between soil and lime by means of tillage is a major factor which determines the speed with which lime alters soil pH.
Other important factors to consider
Pasture renovation
Clover and ryegrass are our most productive pasture species but soil acidification can lead to pastures becoming dominated by poorly productive species, reducing animal production. Therefore, once liming has started, the pasture often needs to be renovated by oversowing with clover and ryegrass.
Grazing management
Grazing management is the key to high pasture use and high pasture use is the basis of profitable grazing industries. Good grazing management also helps maintain good pasture composition. Once low soil pH has been corrected and the pasture has been renovated, it becomes essential to apply good grazing management to use as much paddock-grown feed as possible.
Fertiliser
Pastures growing in low pH soils are usually dominated by poorly producing pasture species so it is a waste of money to apply fertiliser to these pastures. It is also a waste of money to fertilise under-grazed pastures to grow more unused feed. It is only profitable to apply fertiliser to productive pastures that are managed to maintain high pasture use.
Acknowledgements
Numerous people have contributed to the Greener Pastures study between 2003 and 2011.
The project would not have been possible without the support, contributions and dedication from the entire team: John Baker, Don Bennett, Mike Bolland, Graham Blincow, Tess Casson, Len Chinnery, John Crosby ,Patrick Donnelly, Hamish Downs, Ian Fillery, Kevin Gardiner, Gordon Gibbon, Ian Guthridge, ‘Tex’ Hahn, Peter Jelinek, Kathy Lawson, Andrew Lindsay, John Lucey, Corrine Mack, Nola Mercer, John Milligan, Richard Morris, Peter Needs, Leonarda Paszkudzka-Baizert, Bill Russell, Dennis Russell, Greg Sawyer, Neroli Smith, Martin Staines, Frank Treasure, Judy Wills and David Windsor.
We are grateful for the guidance and support by Michael Blake, Laurie Cransberg, Grant Evans, Peter Evans, Dale Hanks, Brynley Jenkins, David Kemp, Ben Letchford, Ian McGregor, Miles Mottershead, Ian Noakes, Peter Oates, Paul Omodei, Ralph Papalia and Victor Rodwell.
We also thank our interstate colleagues for their support and guidance: Roger Barlow, David Chapman, Tom Cowan, Anne Crawford, Tom Davidson, Richard Eckard, Warren Mason and Mark Paine.
We acknowledge funding and support of this project by the Department of Agriculture and Food WA, Dairy Australia and Western Dairy. Additional funding and/or contributions in kind, were provided by the Chemistry Centre (WA), CSIRO Plant Industry, South West Catchments Council and Land and Water Australia.

