Soil acidity and high rainfall pastures
Causes of soil acidification
Conventional agriculture acidifies soil. As soil becomes more acid, productive pasture species disappear and are replaced by species of low agricultural value. It is a waste of money to apply expensive fertiliser to pastures which are inherently poorly productive because they are growing in an acid soil. Soil acidification takes place via two major processes:
Product removal
Plants take up nutrients from soil solution as either positively charged cations (ammonium, sodium, potassium, calcium, magnesium) or as negatively charged anions (nitrate, chloride, phosphate, sulphate, borate, molybdate) and they usually take up more cations than anions. To maintain internal electrical neutrality, roots secrete hydrogen ions to balance the charge of the extra cations taken from soil.
These hydrogen ions are produced from water in the roots dissociating into positive hydrogen ions (acidic) and negative hydroxide ions (alkaline). This makes the adjacent soil more acid and the plant more alkaline, this is the source of ash alkalinity when plant residues are burnt. A diagrammatic presentation is given in Figure 2.
false
The positive charge of the cations now in the roots is balanced by the negative hydroxide ions remaining in roots. The positive charge of hydrogen ions excreted from roots balances the negative charge of anions left in the soil when the extra cations are taken up.
If no product is removed from the paddock, there is no net loss of nutrients and acidification is negligible as plants break down to become soil organic matter. However, in productive agriculture, plants are removed as produce such as grain and fodder, or as animal products such as milk, meat, wool or hides. This acidifies soil.
The nitrogen cycle
Plants and soil organisms — insects, earthworms, algae, fungi, protozoa and bacteria — break down and become part of the soil organic matter. Grazing animals also return organic matter to soil as faeces and urine.
Soil organic matter is a source of protein, energy and nutrients for plants and soil organisms.
They process soil organic matter by physical and chemical means to release some of its nutrients into soil solution, a process known as mineralisation. Urea in urine patches is a source of nitrogen and potassium for both plants and soil organisms.
Plants take up nitrogen from soil as either ammonium or nitrate. Ammonium is among the first forms of nitrogen mineralised from soil organic matter. Ammonium is also added to soil as fertiliser (ammonium phosphates such as MAP and DAP or ammonium sulphate).
Urea, applied as fertiliser or in urine patches, is rapidly converted to ammonium. Ammonium is positively charged and soil constituents — organic matter, clays, oxides — possess both positive and negative surface charge sites. For most soils, the magnitude of the negative charge is much greater than that of the positive charge. Consequently, ammonium is usually retained by negative charge sites and is not usually leached deeper into soil. In very sandy soils, with insufficient negative charge to retain all the ammonium mineralised from organic matter or applied as fertiliser, some can be leached.
In addition, the amount of ammonium and potassium present in urine patches can greatly exceed the capacity of soil to retain these elements, so both can be leached from urine patches. A diagrammatic presentation is given in Figure 3.
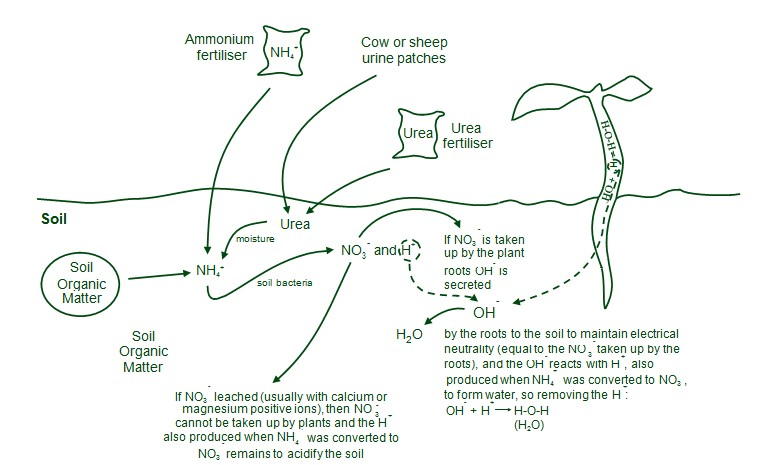
When plant roots take up nitrogen from soil as positive ammonium ions, positive hydrogen ions produced from water in the roots are excreted to maintain electrical neutrality. This acidifies the soil. The hydroxide ions also produced remain in the plant and make it more alkaline — another source of ash alkalinity when plant residues are burnt.
Soil bacteria convert ammonium to nitrate and hydrogen. When plant roots take up nitrogen as negative nitrate ions, hydroxide ions derived from water in roots are secreted into the soil to maintain electrical neutrality. The hydrogen ions also produced remain in the plant and make it more acid. When hydroxide ions are released into the soil, they react with the hydrogen ions to produce water.
Therefore, when ammonium ions are converted to nitrate and hydrogen ions, provided all the nitrate ions are taken up by plant roots, the hydroxide ions secreted in the uptake process consume the hydrogen ions and there is no net acidification of the soil.
However, sandy soils typically have negligible positive charge sites so nitrate ions are readily leached below plant rooting depth. When nitrate is leached, the hydrogen ions remain in the soil and acidify it. Because plants can only take up nitrogen as ammonium or nitrate ions, and ammonium ions are rapidly converted to nitrate ions, leaching of nitrate is a major cause of soil acidification.
