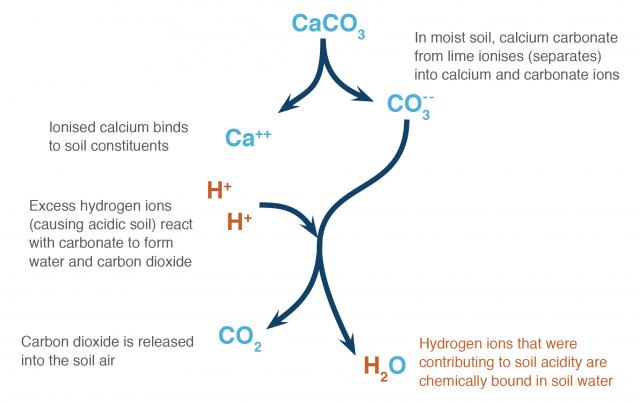
How lime works
Excess hydrogen ions in the soil solution cause soil acidity. When agricultural lime is applied, carbonate from calcium carbonate (or magnesium carbonate) neutralises acid in the soil.
The soil chemistry can be simplified into a few steps:
- In wet acidic soil, calcium carbonate ionises (separates) into calcium and carbonate ions.
- The carbonate ions react with hydrogen ions in the soil solution to form bicarbonate ions.
- The bicarbonate ions react with hydrogen ions in the soil solution to form carbon dioxide and water.
There are more complicated chemical steps in this pathway, but the end result is that the soil has more calcium ions on the exchange surfaces of the soil and carbon dioxide is released into the soil air. Hydrogen ions that were contributing to acidity are then bound in soil water (Figure 1).
Neutralising value (NV)
The key indicators of agricultural lime quality are neutralising value and particle size, regardless of the source.
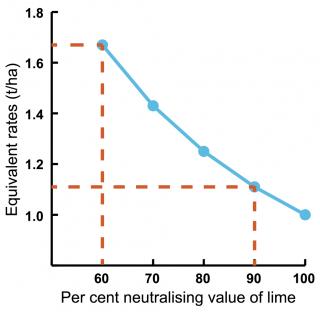
The carbonate content of limesand, limestone or dolomitic lime determines the capacity of the lime to neutralise acidity. Neutralising value is expressed as a percentage relative to pure calcium carbonate, which is given a value of 100%. With higher neutralising value, lime can be spread over a greater area, or less tonnes per hectare (t/ha) used, for the same pH change (Figure 2).
Agricultural lime suppliers should provide results of current laboratory tests detailing the neutralising value (sometimes expressed as NV) of the various particle sizes in their product. These values can be used to calculate the best value lime available.
Particle size
While the quality of agricultural lime deposits can vary widely, high-quality lime is available from limesand, limestone and dolomitic lime sources.
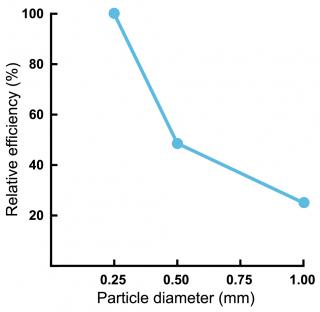
The size of the lime particles determines how quickly the lime can neutralise acid. Lime with a higher proportion of finer particles has a larger surface area to react with the acid in soil. Research shows that finer limes (a high proportion of particles less than 0.5mm) increase pH faster, which is necessary for rapid amelioration of acidic soil (Figure 3).
Suppliers of limestone and dolomitic lime crush and screen their products and suppliers of limesand may screen to remove vegetation if necessary. This processing ensures supply of a consistent product. Suppliers should provide details of the particle size distribution of their product. Farmers should ensure that products contain an adequate proportion of fine particles to meet their needs (Figure 4).