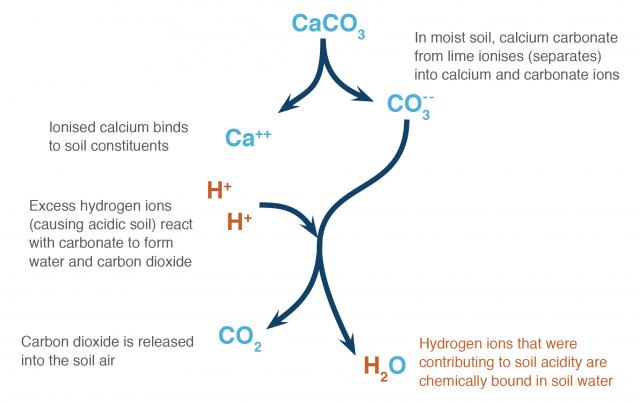
How lime works
Excess hydrogen ions in the soil solution cause soil acidity. When agricultural lime is applied, carbonate from calcium carbonate (or magnesium carbonate) neutralises acid in the soil.
The soil chemistry can be simplified into a few steps:
- In wet acidic soil, calcium carbonate ionises (separates) into calcium and carbonate ions.
- The carbonate ions react with hydrogen ions in the soil solution to form bicarbonate ions.
- The bicarbonate ions react with hydrogen ions in the soil solution to form carbon dioxide and water.
There are more complicated chemical steps in this pathway, but the end result is that the soil has more calcium ions on the exchange surfaces of the soil and carbon dioxide is released into the soil air. Hydrogen ions that were contributing to acidity are then bound in soil water (Figure 1).