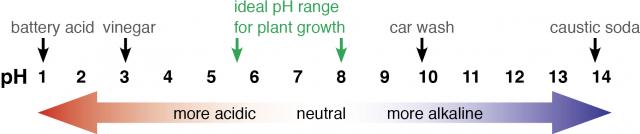
The pH scale
Soil pH is used to indicate the acidity (or alkalinity) of soil, and is a measure of the concentration of hydrogen ions (H+) in the soil solution. pH is measured from 1 (acidic) to 14 (alkaline), with 7 being neutral and is measured on a negative logarithmic scale (base 10).
The lower the pH, the higher the acidity (Figure 1). Most plants are favoured by a pHCa (measured in a 0.01M CaCl2 solution and denoted as pHCa) between 5.5 and 8. Changes in soil chemistry and microbiology when pH is below or above this range adversely impact plant processes resulting in reduced growth and yield.
Because of the logarithmic scale, soil with a pH of 4 is 10 times more acidic than a soil with a pH of 5, 100 times more acidic than a soil with a pH of 6 and 1000 times more acidic than a soil with a pH of 7. This means that a small decrease in soil pH results in a large increase in acidity. For example, there is 2.5 times more acid at pHCa 4.4 than at 4.8. This small, 0.4 of a unit drop from the recommended minimum subsurface pH of 4.8 would result in aluminium toxicity to plant roots in most Western Australian soils.