Summary
- Heavy infestation of aphids caused significant yield loss to canola at this site
- The length of cabbage aphids on spikelets at flowering was proportional to yield loss
- For every centimetre of spikelet colonised about 10% of yield was lost
- Aphid colonisation also reduced seed quality: oil content and seed size
Background and aim
Concerns are increasing that the yield loss of canola to aphids is being underestimated. There are two main mechanisms which cause yield loss;
- Feeding damage by species such as cabbage aphid, which colonise flowering and podding spikelets. Previous trials reported canola to be tolerant of aphid damage with little yield loss unless plants were water stressed. More recent work concluded that aphids can cause yield loss to unstressed canola, however they needed to be present on spikelets from flowering.
- Infection with virus; of particular concern is green peach aphid transmitting beet western yellows virus (BWYV). In 2014, this virus caused widespread damage to South Australian crops and anecdotal evidence indicates an increase in the occurrence of this virus in Western Australia. Yield losses of up to 50% have been recorded when BWYV infection occurred early in plant growth (before flowering).
The aim was to determine the extent of yield loss caused by high levels of aphid infestation on canola in the northern agricultural region and re-investigate management guidelines.
Trial details
The trial was conducted at DPIRD's Geraldton research facility. The design was randomised in two banks with four replicates. Plots were 20m long by 7.2m wide. The middle 3.2m of each plot was harvested such that the outside of each plot acted as a buffer from neighbouring plots.
Treatments
There were four treatments based on different insecticide strategies to control aphids as described below:
- Nil (no insecticide)
- One spray - Sulfoxaflor (Transform®) applied at six leaf stage for control of aphids to stop early virus infection
- Two sprays - Sulfoxaflor (Transform®) at six leaf stage and big bud stage for control of aphids until podding
- Three sprays - control insecticide applied at six leaf stage, big bud stage and flowering for no aphids
Results
Aphid numbers were high at the site. Numbers found on sticky traps increased rapidly from early June to the third week in July and then declined rapidly (Figure 1). Testing of plant leaves in early July indicated that all treatments had BWYV at low levels of infection, ranging from 7.5-12% of plants infected. There was no statistical difference in percentage of plants affected between treatments.
The insecticide sprays applied in the treatments did work to create a range of aphid infestation levels. Biomass cuts confirmed observations of differences in plant growth between treatments. The treatments with fewer insecticide applications produced less biomass and the plants were smaller (Figure 2).
From mid-July the length of the flowering spikelet that was colonised by aphids was measured, these were predominantly cabbage aphids. The applied insecticides reduced aphid infestation (Table 1).
| Date | 16 July | 23 July | 30 July | |
|---|---|---|---|---|
| Treatments | DAS | 79 | 86 | 93 |
| Unsprayed | - | 3.2 | 4.7 | 5.5 |
| One spray: six leaf | - | 2.6 | 3.8 | 4.6 |
| Two sprays: six leaf, big bud | - | 1.7 | 2.4 | 3.1 |
| Three sprays: control (nil aphids) | - | 2.1 | 1.9 | 1.8 |
| LSD | - | 1.1 | 0.8 | 1.2 |
| - | NS | P<0.001 | P<0.001 |
Yield, oil% and seed weight were all reduced when aphids were not controlled; the unsprayed treatment yielded 39% of the control treatment. The trend was for greater yield, oil content and seed weight with each additional application of insecticide although these differences were not significantly different in some cases (Table 2).
| Yield (kg/ha) | Oil % | 1000 seed weight (g) | |
|---|---|---|---|
| Unsprayed | 347 | 38.8 | 2.5 |
| Six leaf spray | 464 | 39.3 | 2.9 |
| Six leaf, big bud stage | 643 | 41.8 | 3.3 |
| Control (nil aphids) | 888 | 43.7 | 3.6 |
| LSD | 294 | 2.2 | 0.5 |
| F Prob | P<0.05 | P<0.05 | P<0.05 |
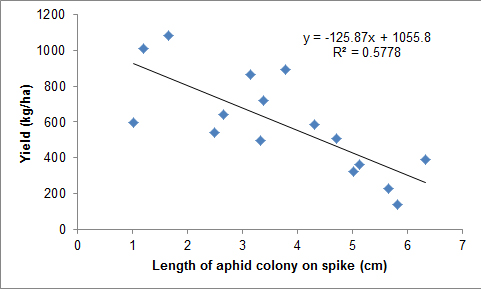
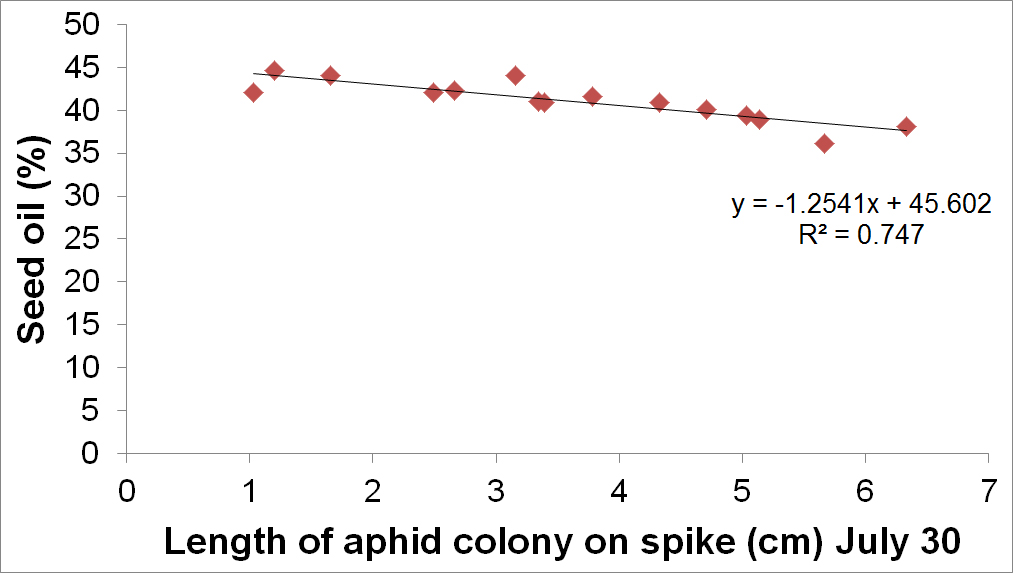
A close relationship was found between the length of the flowering spikelet that was colonised by cabbage aphids and yield, oil and grain weight.
The function yield (kg/ha) = -125.87x + 1055.8 where x = spike length colonised in cm explained yield loss R2 0.58 (Figure 3). This meant that for each centimetre of flowering spikelet that was colonised about 10% of yield was lost.
For oil the function y (oil%) = -1.2541x + 4.15 where x is the average length of spikelet colonised in centimetres (cm) was a good fit (Figure 4).
For seed weight as colony length increased, a decline in 100 seed weight (measure in grams) was observed. For seed weight the function y (100 seed weight) = -0.31x+4.23, where x is the average length of spikelet colonised in centimetres (cm) was a good fit.
It is likely that the main cause of damage was from feeding by cabbage aphid. The functions fitted for yield, oil, and seed weight decline by average length of spikelet colonised will be used to refine management recommendations and provide a more accurate method of assessing the requirement for management of aphid feeding damage.
These results reinforce the need to plan ahead for aphid control. Cultural practices such as maintaining good stubble cover and establishing a thick canopy quickly to minimise bare ground and early aphid landings along with delayed sowing should be considered. Using cultural methods in conjunction with insecticides will extend the useful life of the newly registered insecticide Transform®. Also it should be noted that Transform® is registered to be used no more than twice per season to reduce the risk of resistance developing especially in green peach aphid.
Acknowledgements
Trial number 15GE33. Thanks to Stephanie Boyce, Jo Walker and the Geraldton RSU for trial management and measurements and Brenda Coutts for virus testing.
