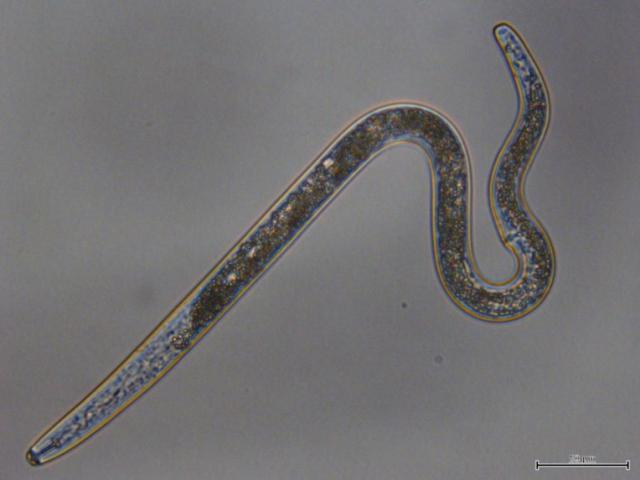
Root Lesion Nematodes: the invisible threat

What are Root lesion nematodes (RLN)?
Root lesion nematodes (RLN; Pratylenchus species) are a microscopic translucent ‘roundworm’ that feed on the roots of crop plants and can cause yield loss. In Western Australia they are concentrated in the top 10cm (root zone) of the soil profile. They can dehydrate to survive the summer in dry soil and roots and become active after rain to invade roots. RLN have multiple breeding cycles each season depending on soil moisture and temperature.
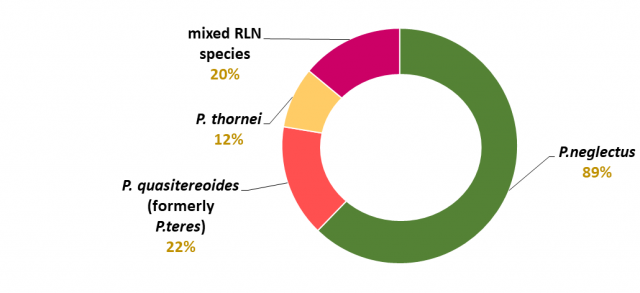
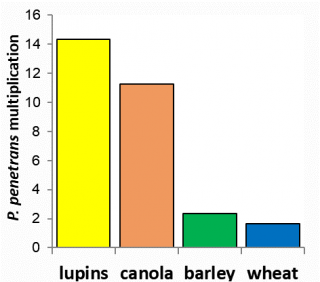
The main species of root lesion nematodes found in broadacre cropping in WA are Pratylenchus neglectus, P. quasitereoides (formerly known as P. teres), P. thornei and P. penetrans (Figure 4). P. quasitereoides creates unique management issues for Western Australian growers. Western Australia is also the only state where P. penetrans is distributed throughout broadacre cropping areas. Here P. penetrans is not common, found in less than 3% of paddocks, but can be very damaging (Figure 3).
RLN are significant pests with a broad host range that includes cereals, oilseeds, grain legumes, pastures and horticultural crops, as well as many broadleaf and grass weeds.
Root-lesion nematodes are found in approximately 6.25 million ha (or ~74%) of the winter broadacre cropping area of Western Australia (WA) (Figure 5). Populations potentially limit yield in at least 54% of these infested paddocks.
Root lesion nematode distribution in WA

Diagnosing RLN in your paddocks
When roots are damaged by RLN they may become less efficient at taking up water and nutrients and less able to tolerate stresses such as drought or nutrient deficiencies. Depending on the extent of damage, the level of RLN and the growing conditions of affected plants, they can partially recover if the rate of new growth exceeds the rate of nematode damage.
The first signs of root lesion nematodes affecting a crop may be poor establishment, stunted growth, poor tillering of cereals and plants wilting despite moist soils. Nematode distribution across a paddock is usually uneven and can result in irregular or wave like crop growth. Symptoms can be confused with and may be exacerbated by less than adequate nutrients and presence of rhizoctonia.

Cereal roots of infested plants will have fewer lateral roots with less branching. Roots may also have areas of browning and the outer root layer or cortex may be damaged and easily disintegrate (Figures 7 and 8). Root lesion nematode damage is difficult to distinguish from damage caused by rhizoctonia which often occurs together with nematodes. Diagnosis of RLN species is not possible from looking at root damage alone and can only be confirmed with laboratory testing. There are two main laboratory services available for Western Australian growers. The diagnostic laboratory (DDLS) at the Department of Primary Industries and Regional Development (DPIRD) offer an in season soil and/or root test for soil borne and also foliar disease diagnosis. PREDICTA® B (provided by Primary Industries and Regions SA) is a preseason soil test to predict potential yield losses from a range of soil borne pests and pathogens but can also be used for in season identification purposes.
For more information on root lesions nematodes refer to GRDC's Tips and Tactics Root Lesion nematodes.
DDLS
Plant pathology is a service provided by the Department of Primary Industries and Regional Development's Diagnostic Laboratory Services (DDLS). They offer foliar, virus, soil borne disease and nematode pest diagnostics services for broadacre crops (cereals, canola and pulses) and pastures. Growers can send samples of good and bad plants along with a submission form for plant disease or pest diagnosis. After diagnosis DDLS can direct growers to Western Australian specialists in pest and disease control for advice on best management practices.
For more information on DDLS click here.
PREDICTA® B
PREDICTA® B is a pre-season DNA based test which is conducted at SARDI (PIRSA). The analyses provided by this service are only for soil borne pathogens and nematode pests. A certified agronomist can collect the soil samples and obtain the results on behalf of growers for a fee. Currently, the PREDICTA® B test can efficiently detect:
- Pratylenchus neglectus, P. quasitereoides and P. thornei from the root lesion nematodes
- Stem nematode
- Cereal cyst nematode
- Take-all (including the oat strain)
- Rhizoctonia
- Crown rot
- Blackspot
Results from the PREDICTA® B service give a pre-season analysis of common disease and nematode pest risk. This allows the grower to make informed decisions about disease management before planting crops. Soil samples can also be submitted during the growing season, as a diagnostic tool to determine if the common nematode and soil borne diseases are present.
Root disease impacts can be difficult to recognise and targeted testing of paddocks can identify situations of increasing or high risk. While PreDicta B® quantifies the level of pathogen DNA in the soil as an indicator of disease risk, it does not predict the disease severity that will occur as this will also be influenced by seasonal conditions and crop choice.
For more information refer to Predicta B.
During the 2017 season, Kylie Chambers (DPIRD Merredin) co-ordinated a survey to investigate the presence and incidence of disease in the eastern wheatbelt. The survey was funded by the National Nematology project with investment from GRDC.
During March- June 2017 a total of 136 soil samples from 128 paddocks were analysed with PREDICTA® B to provide a snapshot of the soil borne disease inoculum and nematode pests in cropping paddocks around the eastern wheatbelt (Kwinana East). A number of new PREDICTA® B tests that are still in development were included. Soil borne disease inoculum and nematode pest levels were sorted into wheat yield loss risk categories of high, medium, low and not detected based on the SARDI risk and population calibrations. The percentage each disease or pest in each category was determined to show the overall risk/ population in the eastern wheatbelt by shire in that season. Several diseases were not detected in the survey including CCN, ascochyta blight, phytophthora root rot, black spot (Phoma Koolunga) and the nematode Pratylenchus thornei.
Three major pathogens known to cause significant crop loss were frequently detected in the survey: crown rot (data not presented), rhizoctonia (data not presented) and the root lesion nematode Pratylenchus neglectus (Table 1). Pratyenchus penetrans was only detected in one sample in the Tammin shire and P. quasitereoides detected twice at low levels in the Merredin shire.
The impact and management of root disease complexes including combinations of crown rot, rhizoctonia and root lesion nematodes also needs to be investigated as there is evidence that yield losses caused by disease complexes are greater than the combined losses of each individual disease.
| Table 1. RLN (Pratylenchus neglectus), detected frequently in the 2017 survey of the eastern wheatbelt with the potential risk of yield loss in a wheat crop. | ||||
| Pathogen | High disease risk | Medium disease risk | Low disease risk | Not detected |
| P. neglectus | 5.1% | 51.5% | 22.1% | 21.3% |
* risk categories are a guide only, may be subject to regional and seasonal differences, and may be revised over time.
Symptoms of root lesion nematode have been detected in the eastern wheatbelt during the 2018 season.
Full details of this research can be found in Characterising soil borne disease risk in the eastern wheat belt of Western Australia and national significance of major diseases.

Managing RLN
In WA, RLN are widespread across the grain belt (Figure 5) and losses are potentially large. In 2016, 63% of NVT paddocks sampled in WA were infested with RLN; 83% of infested paddocks contained P. neglectus and 23% P. quasitereoides. Potential yield losses in cereals can be up to 50% if beginning of season levels are >15 RLN/g soil.
Currently, there are no economically viable nematicides available to control RLNs in broadacre cropping. Management is based on crop rotation and variety selection. A resistant crop or variety will not cause RLN levels to increase over a growing season whilst a susceptible crop or variety will cause an increase. The resistance of crops and varieties depends on the root lesion nematode species present (Figure 10, 11 and 12). Susceptibility of wheat and barley varieties to P. neglectus and P. quasitereoides can be utilised for RLN management by growers (refer to Western Australia’s wheat and barley guides).
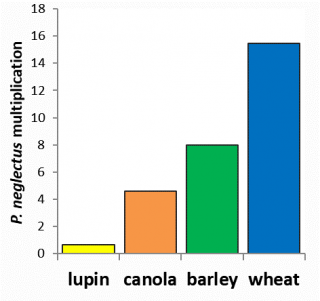
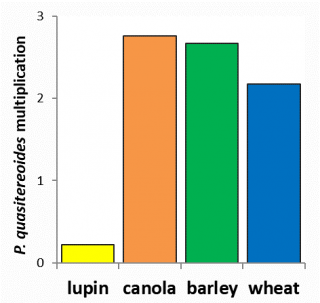
Increased understanding of the effects of rotational crops on RLN densities improves our ability to determine their potential effects on subsequent cereal crops. Likewise an understanding of the RLN tolerance of current wheat, barley, pulse and oilseed varieties helps estimate the potential economic effects of RLNs in WA and aids in appropriate choices for infested paddocks.
In cereal crops paddock symptoms include uneven or wavy growth, less tillering, yellowing of the lower leaves and early wilting. Above ground RLN symptoms had not been observed in canola plants and it has been assumed that canola yields were not impacted by RLN infestation. However, with an increasing utilisation of canola in WA cropping rotations and increasing RLN levels in the Western region this needed more investigation.
Sarah Collins and Carla Wilkinson (DPIRD) have conducted a series of trials, 2015-2017, to determine the field resistance (impact of crop on nematode population) and tolerance (impact of nematode on crop yield) of triazine tolerant (TT) canola varieties to root lesion nematodes P. neglectus and P. quasitereoides in different Western Region Agzones, Wongan Hills (Agzone 2; P. neglectus) and Gibson (Agzone 6; P. quasitereoides). While numerous wheat, barley and lupin varieties were also tested in these trials, results are only shown at a crop level.
Crop resistance varied between the RLN species tested (Figure 10, 11 and 12). Lupin varieties reduced P. neglectus and P. quasitereoides populations over the season and are therefore a good choice to manage these RLNs when they are at levels that could cause significant yield loss in other crops. For both RLN species there are differences in susceptibility between varieties which can be utilised by growers to limit RLN build up to prevent potential yield loss in grain crops. For wheat and barley, resistance information can be found in our WA variety guides and can be used to help growers make suitable rotational choices in infested paddocks.
Canola is commonly regarded as a good break crop to reduce soil borne diseases in cereal rotations, particularly where rhizoctonia is an issue, but canola is likely to cause an increase in RLN levels. In these concurrent trials, canola increased both P. quasitereoides and P. neglectus levels by an average of 3 to 5 times, reflecting results from previous DPIRD research.
| Variety | P. neglectus (Wongan Hills) | P. quasitereoides (Gibson) | ||||||
| Pi* | Pf⌂ | Multiplication | Pi* | Pf⌂ | Multiplication | |||
| ATR Cobbler | 11 | 45 | 3.7 | a◊ | 6 | 10 | 3.2 | a◊ |
| ATR Snapper | 10 | 40 | 4.2 | ab | 8 | 14 | 2.7 | a |
| Telfer TT | 8 | 41 | 4.4 | ab | 5 | 12 | 3.6 | a |
| Sturt (TT) | 10 | 49 | 4.9 | ab | 5 | 12 | 3.2 | a |
| ATR Stingray | 11 | 53 | 5.6 | b | 5 | 13 | 2.9 | a |
| Av. | 10 | 45 | 4.6 | 6 | 12 | 3.1 | ||
Note. *Pi = average RLN level at the beginning of season. ⌂Pf = average RLN level at the end of season. ◊ = Different letters denote significant difference in multiplication between varieties from multiple comparisons (at the significance level of 0.05) using Fisher’s Protected LSD.
Canola variety resistance to RLN (nematode impact)
All canola varieties increased the RLN levels over the season but the degree of P. neglectus multiplication was affected by the variety grown with ATR Stingray was the most susceptible variety increasing the RLN population more during the season than ATR Cobbler (Table 2). P. quasitereoides multiplication was not significantly different between canola varieties indicating that there was no difference in resistance between the varieties tested.
Canola tolerance to RLN (yield impact)
Yield impacts caused by RLN infestation were large with an average loss of 16% at both Wongan Hills (398 kg/ha) and Gibson (275 kg/ha) due to P. neglectus and P. quasitereoides, respectively. Yields of all varieties were reduced when RLN was medium to high at the time of sowing; average of 17 P. neglectus/g soil at Wongan Hills and 11 P. quasitereoides/g soil at Gibson (Figures 7 and 8). Variety tolerance to RLN was not consistent between RLN species.
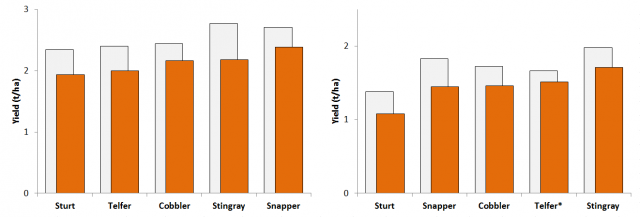
Full details of this research can be found in The invisible threat:Canola yield losses caused by root lesion nematodes in WA.
Are rotations offering breaks for RLN
Pastures and other legumes
Some pastures are effective for reducing root lesion nematode (RLN; P. neglectus and P. quasitereoides) populations in a season, but others can greatly increase RLN levels.
In WA, proven resistance of serradella pastures to our common RLNs, P. quasitereoides and P. neglectus, offers growers with infested paddocks new management options. Serradella can be used as a cleaning phase for RLN as well as introducing nitrogen naturally to the soil. Biserrulla and subterranean clover also show potential to reduce RLN but more experimentation is necessary to confirm their resistance. Clovers on the other hand vary greatly in resistance to P. neglectus from very susceptible to moderately resistant so clover type and variety choice needs to be handled with care.

A range of pasture crops and some specific pasture varieties were tested in DPIRD glasshouse trials in 2007, 2008 and 2017 and have shown good levels of resistance to WA’s common RLNs; P. quasitereoides and P. neglectus. For cropping rotations using susceptible crops like wheat, barley or canola, these pastures will limit RLN multiplication and reduce the chance of damage to a subsequent susceptible crop. Serradella varieties have proven to be resistant in the glasshouse to P. neglectus and in grower paddocks to P. quasitereoides (Table 3). In fact, P. quasitereoides population reduction under serradella was better than lupin in the same paddock in Gibson (Table 3, Lupin data not shown). Casbah biserrulla and Dalkeith subterranean clover showed good levels of resistance to P. neglectus in glasshouse experiments conducted in 2017 (Figure 15). This indicates that in cropping rotations, Casbah biserrulla and Dalkeith subterranean clover are likely to decrease P. neglectus populations in a season, but they may also increase numbers in some circumstances.
Conversely, a number of clover species significantly increased P. neglectus populations in all three glasshouse experiments. Species from popular pasture types, Balansa, Persian and Gland clovers for example, were as bad as the susceptible wheat control in terms of increasing P. neglectus populations in their root systems (Figure 15). Therefore these clovers are likely to contribute to increased P. neglectus populations in cropping paddocks and should be avoided in rotation for infested paddocks.
| Location | Season | Paddock | Before Serradella RLN/g soil | After Serradella RLN/g soil | Multiplication |
| Pingelly | 2015 | 1 | 21 | 4 | 0.16 |
| 2015-16 | 2 | 45* | 1 | 0.03 | |
| Gibson | 2013 | 1 | 4 | 1 | 0.19 |
* Sample collected at the end of the previous season directly after oats crop.

For further information on this work refer to Oats, pastures and other legumes – Are rotations offering breaks for root lesion nematodes or crown rot?
Oats
Traditionally it has been thought that oats are the most resistant of the main cereal crops grown in WA but currently grown varieties had not been tested in field trials. Carla Wilkinson and Sarah Collins investigated the use of oats as a break crop for RLN in field and glasshouse trials supported by COGGO (2016 and 2017).
This project showed that oats are not a good break crop in paddocks infested with either RLN species P. neglectus or P. quasitereoides as they are likely to cause nematode levels to increase which may then cause issues in subsequent crops. There is also evidence that P. quasitereoides can cause yield loss in oats. In general oats were more resistant than wheat to P. neglectus and more susceptible than wheat to P. quasitereoides.
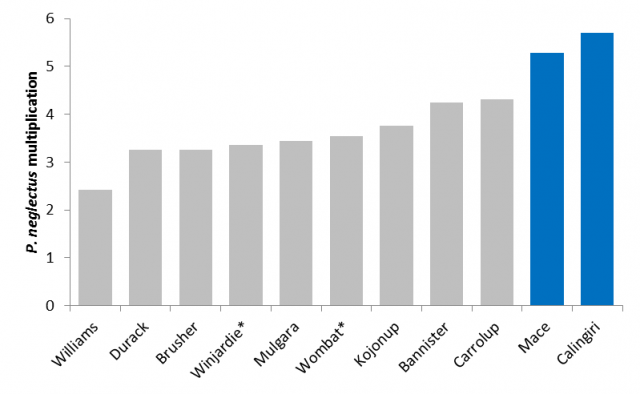
P. neglectus and oats
All oat varieties tested were susceptible to P. neglectus and will likely cause levels in an infested paddock to increase over a season which may affect yield and growth of a subsequent crop. In general, oats were more resistant than wheat and barley to P. neglectus and may be a better cereal rotation option to manage P. neglectus levels. There was little difference in resistance between oat varieties to P. neglectus in field trials (Figure 16). In glasshouse trials oats were generally less susceptible than wheat and barley but more susceptible than lupin and serradella pastures.
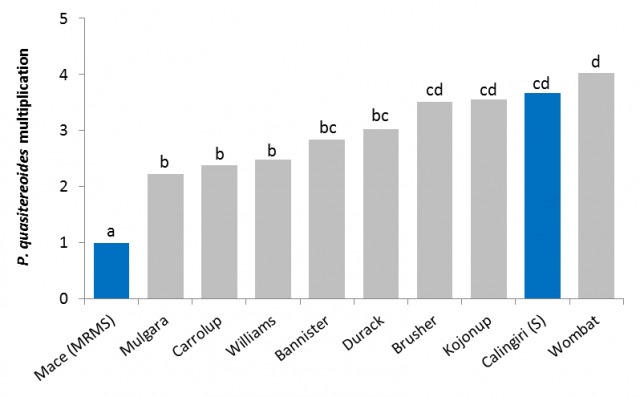
P. quasitereoides and oats
All oat varieties tested were more susceptible to P. quasitereoides than the most commonly grown wheat variety in Western Australia, Mace (moderately susceptible to moderately resistant, Figure 17). This means that growing any variety of oats is likely to cause a greater increase in P. quasitereoides levels in a paddock than growing Mace. Results from two field trials indicate that Williams and Mulgara may be the most resistant oat varieties to P. quasitereoides but this needs further testing. P. quasitereoides caused grain yield loss in oats of 0.23 t/ha for every RLN/g soil in Beverly in 2017. Estimation of potential yield loss from RLN’s, particularly P. quasitereoides requires further research.
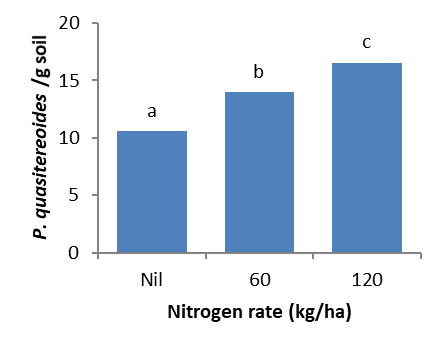
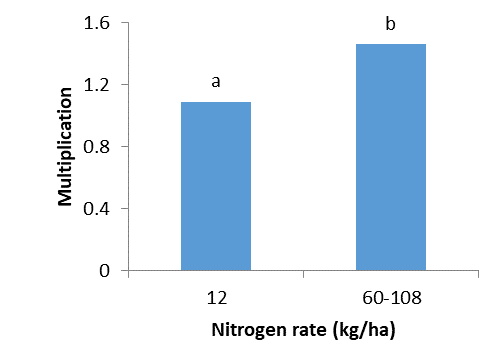
Nitrogen fertiliser and root lesion nematode interactions
Trials at Westdale and Dumbleyung in 2017 measured the effect of nitrogen forms, application timing and rates on crop production and root lesion nematode (RLN) levels.
Key Points:
- Nitrogen form, rate or timing did not reduce P. quasitereoides numbers in susceptible wheat crops compared to plots where less nitrogen was applied.
- Higher rates of nitrogen fertiliser increased root lesion nematode (P. quasitereoides) multiplication. This is likely due to better root growth which increased the available food for root lesion nematodes.
- Nitrogen fertiliser increased grain yield and/or protein producing higher returns.
Increasing crop nutrition, especially nitrogen, has been shown to decrease yield loss caused by P. thornei, another root lesion nematode species. There is also evidence that fertilisers containing or releasing ammonium are effective in suppressing plant parasitic nematodes in soil whilst nitrate is less effective. It’s proposed that inorganic ammonium fertilizers may kill RLN’s moving in the soil profile if they are directly exposed to ammonia during the fertiliser’s break down. Reduction in plant parasitic nematodes after application of these fertilisers may also be due to a change in microbial activity stimulated by increased ammonium in the soil.
In 2017, field trials in Westdale and Dumbleyung investigated the effect of nitrogen rate, timing and form on P. quasitereoides levels. The hypothesis was that high rates of nitrogen as urea at seeding would decrease root lesion nematode infestation of plants compared with nitrogen applied as nitrate or applied at lower rates.
| Treatment | Nitrogen (kgN/ha) applied AS* | Nitrogen (kgN/ha) applied 8 WAS** | Grain yield (t/ha) | Protein % | Wheat grade | Gross return net of nitrogen costs ($/ha) |
| Westdale site | ||||||
| Nil | - | - | 4.3 | 8.9 | ASW1 | $1,079 |
| Urea upfront 60 kgN/ha | 60 | - | 4.8 | 8.8 | ASW1 | $1,175 |
| Urea split 60 kgN/ha | 30 | 30 | 5.0 | 8.7 | ASW1 | $1,220 |
| Urea upfront 120 kgN/ha | 120 | - | 5.5 | 8.8 | ASW1 | $1,321 |
| Urea split 120 kgN/ha | 60 | 60 | 5.2 | 9.3 | ASW1 | $1,240 |
| FlexiN upfront 60 kgN/ha | 60 | - | 4.6 | 8.9 | ASW1 | $1,132 |
| FlexiN split 60 kgN/ha | 30 | 30 | 4.8 | 8.8 | ASW1 | $1,177 |
| FlexiN upfront 120 kgN/ha | 120 | - | 4.8 | 8.8 | ASW1 | $1,160 |
| FlexiN split 120 kgN/ha | 60 | 60 | 5.3 | 8.8 | ASW1 | $1,280 |
|
| ||||||
| Dumbleyung site | ||||||
| Basal 12 kgN/ha | 12 | - | 2.6 | 9.4 a | ASW1 | $634 |
| Sowing 60 kgN/ha | 60 | - | 2.8 | 11.0 b | APW1 | $680 |
| Sowing 108 kgN/ha | 108 | - | 2.9 | 12.1 c | H2 | $692 |
| Split 60 kgN/ha | 36 | 24 | 2.8 | 11.1 b | APW1 | $682 |
| Split 108 kgN/ha | 60 | 48 | 2.9 | 12.0 c | H2 | $680 |
| Later 60 kgN/ha | 12 | 48 | 2.9 | 10.9 b | APW1 | $684 |
| Later 108 kgN/ha | 12 | 96 | 2.7 | 12.3 c | H2 | $643 |
No nitrogen rate, form and/or timing of application resulted in a reduction in P. quasitereoides in a susceptible wheat crop. Conversely, at both sites increased nitrogen application, regardless of form, increased P. quasitereoides levels (Figures 18 and 19). Increased nitrogen was also found to increase wheat root growth. It is likely that greater root growth provided a larger food source for the nematodes which led to increased levels. Extra nitrogen also increased yield and/or grain quality in P. quasitereoides infested paddocks (Table 4). Gross profit margin was maximised with largest upfront applications of either Agstar or Urea (Table 4).
These trials showed that applying extra nitrogen, to lessen potential crop losses in P. quasitereoides infested paddocks may also increase root lesion nematode levels due to a larger food source from increased root production. Higher levels of nematodes remaining in the soil at the end of season may negatively impact yield and growth of subsequent crops. However, applying higher rates of nitrogen may increase yield, grain quality and profit in the current season in infested paddocks. For plant parasitic nematodes, current best practice involves testing poor production areas for soilborne pests and diseases to inform management options, such as sowing resistant break crops and more tolerant varieties.
Deeper deep ripping – what are the effects on weeds and RLN?

Soil renovation techniques, such as deeper deep ripping, provide crop benefits by improving the soil condition allowing plant roots better access to water and nutrients. There is a requirement for more research into techniques to achieve good crop establishment and weed control post soil renovation to capitalise on the benefits of deeper deep ripping (DPIRD).
This season, Bonnie Jupp and Bindi Isbister (DPIRD) are conducting a trial at a property east of Geraldton to investigate the influence of soil renovation on crop and weed establishment in a root lesion nematode P. neglectus infested paddock. The grower renovated the paddock before commencement of the season using deeper deep ripping, but has left nine 18m width strips un-ripped. This will allow research to determine if crop and weed establishment differed between the renovated and un-renovated areas.
P. neglectus levels will also be measured in 9 transect plots of 18m length in both renovated and renovated during the season. This will allow a comparison of RLN levels and population change over a season between the renovated and un-renovated strips.
Projects in 2018
In 2018, DPIRD is continuing to work in partnership with GRDC and agronomy groups to investigate the effect of P. quasitereoides on yield of wheat, barley, canola and legumes. We have established trials in Kojonup, Williams and Westdale to set-up plots with differential nematode levels in 2018 and in 2019 we will test a range of varieties for yield loss.
DPIRD is also co coordinating two one year resistance trials comparing the resistance to P. neglectus of a number of wheat, barley and triticale varieties with their resistance in the eastern states. From these comparisons we aim to see if this RLN species multiplies similarly in WA soils compared to other regions.
Our DPIRD nematology group are also collaborating with other researchers conducting trials investigating:
- break crop options for managing RLN and rhizoctonia in highly infested paddocks
- break crop options for managing RLN only in a highly infested paddock
- the effect of long term pasture management on RLN levels
- the interaction between RLN and crown rot on wheat
- the effect of nitrogen and potassium RLN levels on RLN multiplication and wheat grain protein when grown on lupin and canola stubble (Summit Fertiliser and Facey Group).
Upcoming field days where you can learn about RLN
- DPIRD Pest and disease diagnosis workshop 20 -23rd August. For more information refer DPIRD's 2018 crop disease and insect identification workshop.
- Facey Group Spring Field Day Yearling 12th September, contact the Facey Group on ph: 9888 1223 for further information.
- Pathology/agronomy field walk/workshop in Brookton 17th September or contact Bec Swift (DPIRD) on ph: +61(0) 8 9690 2081 for more information or to RSVP.