Observing the life cycle of snails in vineyards
Introduction
A 12 month study observing brown garden snail (Cornu aspersum [Muller, 1774] Helicidae) activity within a Margaret River vineyard was recently completed. The aim was to determine the reproductive state of the population over the year by measuring albumen gland and shell size of individuals. This complements the snail baiting demonstration article published in the December 2021 edition of the Wine Industry Newsletter, that demonstrated the efficacy of different snail bait products.
Background
Brown garden snails can lay up to six batches (Bezemer & Knight 2001) (30-120 eggs) per year and the breeding activities happen most frequently in warm and moist conditions (Dekle, et al 2003). Snail eggs can hatch within 10 days or up to three weeks (Crowell 1984), depending on temperature (Dekle, et al 2003). Brown garden snails tend to reach sexual maturity one to two years from hatching but if conditions are highly favourable can be shortened to six months (Crowell 1984).
The albumen gland in snails relates to the animal's breeding viability. It produces a nutritive secretion onto fertilised eggs and consequently will increase in size before a snail lays eggs (Baker 1988). Contrary to this, the lack of gland development indicates inactive egg production (Baker 1988).
Twenty large brown garden snails (shell width 15mm - 31mm) were collected from an untreated vineyard block located in Margaret River each month (March 2021 to February 2022), submerged in water and preserved in a 70% ethanol solution prior to dissection. Dissection of individuals was to determine peak reproductive activity, thus the best timing for snail management. Shell width and albumen gland length were measured using methods established by Baker (1988).
Results
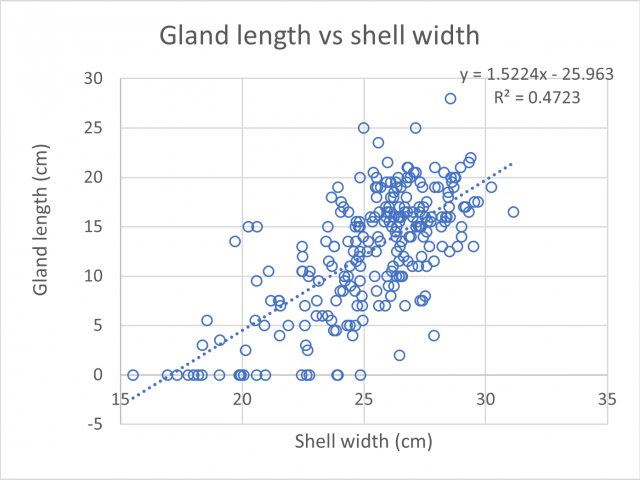
There was a positive relationship between shell width and albumen gland length (Figure 1), meaning during this period of observations it was found that the bigger the snail the larger the gland, indicating large individuals are able to reproduce. In contrast, the shell widths of individuals without a detected albumen gland were all under 25mm.
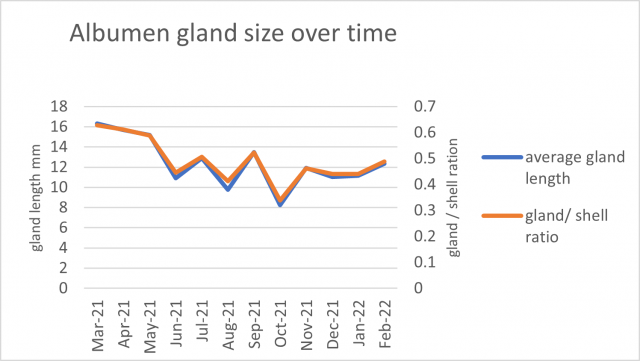
The albumen gland length and its ratio to shell width was greatest in March 2021, followed by a decrease until October before increasing again (Figure 2). The March peak indicates the greatest number of individuals were laying eggs at that time. The average size of the dissected brown garden snails for each period was relatively consistent. It was noted during collection that individuals >25mm (large) in diameter were more difficult to locate in June, July and August compared to smaller snails.
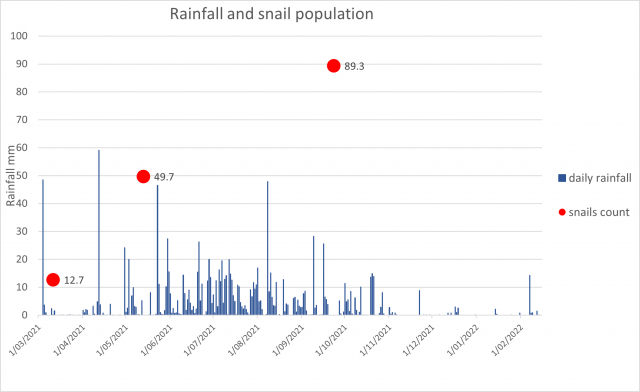
Population data (snails from a single panel within a row) combined with daily rainfall data demonstrates a clear increase in numbers from March (12.6 snails per panel) to September (89.3 snails per panel) which correlates with increases in rain events and rainfall accumulation.
Seasonal observations
Brown garden snails were observed actively laying eggs in the beginning of March (2021). Observed egg laying coincided with a significant rainfall event. Greater populations of ‘small’ active snails were noted in winter, compared to other periods of the year. Most brown garden snails were located on vine trunks in winter with the larger specimens noted to be in an aestivation (dormancy) phase.
In spring, populations tended to move into the vine canopy. Increased variability in size was also visually observed in the population during the spring period.
During summer, aestivating brown garden snails were predominately observed in the ground cover beneath the vines. Interestingly these aestivating individuals upon dissection were found to have large albumen glands, leading to the assumption that they could opportunistically reactivate and commence egg laying under favourable conditions.
Discussion
The size of the brown garden snail albumen gland was greatest in March. However, an enlarged albumen gland was present in most larger brown garden snails ( >25mm) throughout the year, which indicates that adults are able to lay eggs when favourable conditions occur (i.e. high moisture levels). Initial results from complimentary research in South Australian orchards and vineyards support these findings.
Within the vineyard, observations of egg laying in March and increases in visible populations in September may suggest shorter development periods when conditions are wet. Results suggest 2021 was a favourable year for brown garden snails to increase populations at the Margaret River vineyard.
Conclusion
Brown garden snails are opportunistic breeders, whose populations can increase rapidly when conditions are suitable. Without timely and effective baiting, snail populations can significantly increase and become a major management issue causing damage to buds early in the season, defoliating vines throughout the season and contaminating fruit at harvest. Regular monitoring, timely baiting (when snails are active and before laying eggs) and adequate application rates are important to suppress snail populations and to avoid heavy infestations.
Due to the limitations of this study; being from a single site, a single year and no replicates, further research is required to advance the understanding of snail biology in WA vineyards and investigate the opportunities for new management strategies.
For more information on this work, contact Yu-Yi Liao, Technical Officer or Richard Fennessy, Research Scientist.
Acknowledgements
This activity is funded via the Wine Australia Regional Program which is administered by Wines of WA. Support from Mike Sleegers at Cowaramup Agencies and AHA Viticulture in providing access to a site and bait products is also greatly appreciated. Technical advice and support were received from DPIRD staff Svetlana Micic (Entomologist) and Andrew van Burgel (Applied Statistician). Dr Michael Nash and Dr. Sally Foletta are thanked for their input and review of this article.
Reference
Baker, G.H. 1988, 'The Life-History, Population Dynamics and Polymorphism of Cernuella-Virgata' (Mollusca, Helicidae)' Aust J Zool, 1988, 36, pp. 497–512, viewed 18 May 2022.
Bezemer, T.M. & Knight, K.J. 2001, 'Unpredictable responses of garden snail (Helix aspersa) populations to climate change' viewed 23 May 2022.
Crowell, H.H. 1984, ‘The brown garden snail’, Ornamentals Northwest Archives , Jan.-Feb.-Mar., Vol. 8, Issue 1, pp.11-13, viewed 12 May 2022.
Dekle, G.W. & Fasulo, T.R. 2003, ‘Brown garden snail’, University of Florida, Department of Entomology and Nematology, Available viewed 24 May 2022.